Background: Platelet component (PC) transfusion is required for allogeneic hematopoietic stem cell transplantation (HCT) recipients. Contamination with infectious pathogens (bacteria, viruses, or protozoa) and T-cells is a risk factor for transfusion-transmitted infection (TTI) and transfusion associated graft-versus-host disease (TA-GVHD). Pathogen inactivation (PI) treatment of PC with amotosalen-UVA (PI-PC, INTERCEPT Blood System, Cerus Corp) in platelet additive solution (PAS) without bacterial screening, gamma irradiation, CMV serology, and with 7-day storage has been the standard of care in Switzerland since 2011 to manage risk of TTI and TA-GVHD. PI-PC have replaced conventional PC (C-PC) prepared in PAS with gamma irradiation and 5 day storage. We previously reported platelet usage in two consecutive five year periods at the University Hospital of Basel. Mean PI-PC dose was higher (3.0 vs. 2.8 x 1011, p=0.001) and mean storage duration longer (4.2 vs. 3.4 days: p=0.001) than with C-PC. PC expiration wastage was reduced with 7-day PI-PC storage vs. 5-day storage (1.5% vs. 8.7%). For HCT recipients, days of PC support; PC use per patient; and RBC use per patient were similar, despite 24.3% lower corrected count increments (CCI) with PI-PC. Now, we report the impact of these observations on treatment related mortality (TRM) and overall survival (OS) 100 days after HCT.
Patients and Methods: A two-period retrospective cohort study was conducted to evaluate PI-PC impact on outcomes of consecutive first allogeneic HCT recipients from January 2006 to December 2010 (Period 1, P1), when gamma-irradiated apheresis C-PC were used, and Period 2 (P2) from January 2011 to December 2017, when apheresis and whole blood-derived PI-PC were used. The review utilized 100-day OS and 100-day TRM to determine the impact of PI-PC on HCT outcomes. Descriptive statistics were used for continuous variables and log-rank analysis for survival outcomes. Univariate analysis was performed using Pearson χ2 statistics. Multivariate Cox regression modelling analyses included: PC period (P1, P2), donor match (HLA identical/twin, matched related, matched unrelated), disease state (early, intermediate, late), and conditioning regimen (reduced intensity, myeloablative) with TRM as the outcome. This was an IRB approved single-center analysis.
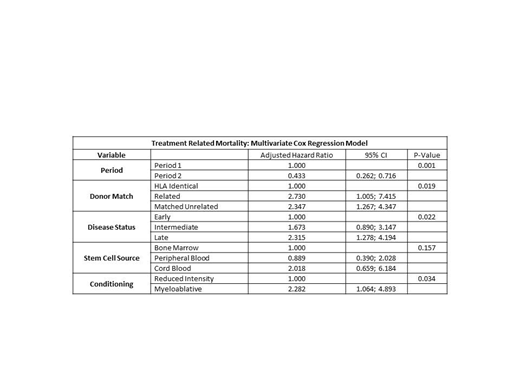
Results: In P1 and P2, 256 and 557 consecutive first-time allogeneic HCT recipients were included, respectively. By univariate analysis, the distribution of European Group for Bone Marrow Transplantation (EBMT) risk scores (grouped 0-2, 3-4, 5-7) and mean patient age were higher during P2 (p = 0.001 and p <0.001, respectively). Primary disease status (p = 0.039); stem cell source (p <0.001); GVHD prophylaxis with ATG (p <0.001); total body irradiation (p <0.001); and conditioning regimen (p <0.001) were different between P1 and P2. Donor match (p=0.084) and disease status (p = 0.628) were similar in P1 and P2. TRM at day 100 post HCT was significantly less (31/557, 5.5%) for PI-PC recipients in P2 vs. C-PC recipients in P1 (37/256, 14.5%, p<0.001). Overall proportion of survivors at day 100 post HCT was significantly greater for PI-PC recipients (507/557, 91.0 %) compared to C-PC recipients (209/256, 81.6%, p <0.001). By multivariate Cox regression analysis, P2 with PI-PC component support was associated with improved TRM (p = 0.001; adjusted hazard ratio 0.433; 95% confidence interval: 0.262, 0.716). Donor match (p = 0.019), disease state (p = 0.022), and myeloablative conditioning (p = 0.034) were associated with significantly poorer TRM (Table). Stem cell source was not significant (p=0.157) in the model. Hemorrhage was reported as cause of death in 1/50 (2.0%) patients during P2 with PI-PC and 4/47 (8.5%) patients during P1 with C-PCs.
Conclusions: Universal implementation of PI-PC in routine with extended storage to 7 days in P2 was associated with reduced TRM and better overall survival 100 days post HCT, despite transplantation of older patients with higher EBMT risk scores. Multivariate analysis revealed an adjusted hazard ratio of 0.433 (95% C.I. 0.262, 0.716) for TRM by 100 days, suggesting better outcomes in P2. This retrospective analysis at a single site indicated that PI-PC treated with amotosalen /UVA stored up to 7 days did not have a negative impact on TRM and OS in HCT recipients, and was an integral part of improving clinical outcomes at our institution.
.
Heim:Novartis: Research Funding. Irsch:Cerus Corporation: Employment, Equity Ownership. Lin:Cerus Corporation: Employment, Equity Ownership. Benjamin:Cerus Corporation: Employment, Equity Ownership. Corash:Cerus Corporation: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal