#: AMZ and NAP contributed equally to this work
Introduction: Anthracycline- and cytarabine-based induction chemotherapy (IC), known as "7+3", has been the standard of care for fit patients (pts) with AML for over 45 years. While various aspects of IC admissions, such as use of mechanical ventilation and dialysis, admission to intensive care units (ICU), and IC-related mortality have been reported in clinical trial setting, little is known about experience and outcomes with 7+3 therapy in real-world settings. We conducted a large population-based retrospective cohort study to assess inpatient care delivered in the US for AML pts receiving 7+3 including IC-related death rates and their predictors.
Methods: We used the Premier Healthcare Database, which includes data from geographically diverse, non-profit, nongovernmental, community and teaching hospitals in the US. Premier captures information from 25% of all inpatient admissions across all ages and payor groups. The database includes demographics, diagnoses, and detailed information on diagnostic tests and the use of drugs, procedures, and other inpatient resources, with dates relative to admission. We selected adult (age ≥18 years) AML pts who received 7+3 during their first recorded inpatient stay between 2010 and 2017. Pts were excluded if they 1) were diagnosed with acute promyelocytic leukemia; 2) received "non-7+3" chemotherapy; or 3) had an inpatient length of stay exceeding one year. We defined antifungal prophylaxis (px) as receipt of non-topical agents within the first 14 days after 7+3 initiation and stratified as mold-directed vs not (fluconazole only). Hospitals were categorized based on the mean number of AML pts included in the study per year as low- (<4), medium- (4-8) or high- (≥9) volume, respectively. Aims of this study were to identify treatment and resource utilization patterns in a US population-based dataset of AML pts stratified by hospital volume.
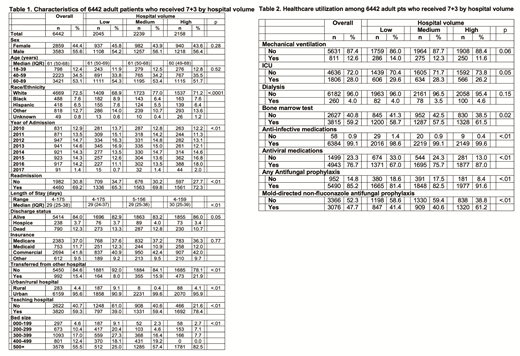
Results: A total of 6,442 AML pts from 313 hospitals who received 7+3 were included. Median age was 61 years (interquartile range [IQR]: 50-68 years; 53.1% age ≥ 60 years); 56% of pts were male (Table 1). Median length of stay was 29 (IQR: 25-38) days with percentages of in-hospital death and discharge to hospice of 12.3% and 3.7%, respectively (total 16.0%). In-hospital death or discharge to hospice were lower in high-volume hospitals (14.0%) compared to low- (17.1%, p<.01) and medium- (16.8%, p=.01) volume institutions (Table 1). Among pts who died in hospital, 76.8%, 19.9% and 3.3% died in first 30 days, days 31-60, and more than 60 days from first day of 7+3, respectively. Pts in low volume hospitals (81.7%) were more likely to die in the first 30 days of IC compared to those treated in high volume hospitals (70.4%, p<.01).
During their inpatient stay, 12.6% and 4.0% of pts required ICU admissions with mechanical ventilation and dialysis, respectively, with no difference by hospital volume (Table 2). Only 59.2% of pts had claims for bone marrow aspirate/biopsy (BMA) during their inpatient stay. 99.1% received anti-infective medications, and 76.7% received anti-viral medications. Pts in high-volume hospitals were more likely to undergo BMA (p=.02), anti-infective medications (p<.01), and anti-viral medications (p<.01). Of all pts, 14.8% received no antifungal px, while most pts (52.3%) received only fluconazole (non-mold directed) px. Proportions of any antifungal prophylaxis and mold-directed antifungal px were significantly higher for pts treated in high-volume hospitals (91.6% and 61.2%) than those treated in low-volume (81.4% and 41.4%) or medium-volume (82.5% and 40.6%) hospitals (all comparisons significantly different with p<0.01).
Conclusions: To our knowledge, this is the largest population-based study to examine practice patterns and induction mortality for AML pts receiving "7+3" in the US. One of seven pts receiving 7+3 died in the hospital during induction or was discharged to hospice. We observed high use of intensive resources. IC-related mortality, use of any antifungal px, and use of mold-directed antifungal px were significantly better in high-volume hospitals compared to low- and medium-volume hospitals. Further analyses are ongoing to examine other predictors of IC-related deaths. Improved understanding of factors that predict induction-related mortality with 7+3 is vital to develop strategies that improve patient outcomes.
Zeidan:Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Ariad: Honoraria; Jazz: Honoraria; Medimmune/AstraZeneca: Research Funding; ADC Therapeutics: Research Funding; Acceleron Pharma: Consultancy, Honoraria, Research Funding; Celgene Corporation: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Trovagene: Consultancy, Honoraria, Research Funding; BeyondSpring: Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Cardinal Health: Honoraria; Seattle Genetics: Honoraria; Daiichi Sankyo: Honoraria; Astellas: Honoraria; Otsuka: Consultancy, Honoraria, Research Funding; Agios: Honoraria. Podoltsev:Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Research Funding; Astellas Pharma: Research Funding; Daiichi Sankyo: Research Funding; Sunesis Pharmaceuticals: Research Funding; Kartos Therapeutics: Research Funding; Jazz Pharmaceuticals: Research Funding; Pfizer: Research Funding; Astex Pharmaceuticals: Research Funding; CTI Biopharma: Research Funding; Celgene: Other: Grant funding, Research Funding; Genentech: Research Funding; AI Therapeutics: Research Funding; Samus Therapeutics: Research Funding; Arog Pharmaceuticals: Research Funding. Huntington:Celgene: Consultancy, Research Funding. Neparidze:Janssen Scientific Affairs, LLC: Research Funding; Eidos Therapeutics: Other: Member of Independent Diagnostic Committee; MMRF/Synteract: Membership on an entity's Board of Directors or advisory committees. Gore:Celgene Corporation: Consultancy, Research Funding. Ma:Celgene Corporation: Research Funding. Davidoff:Celgene Corporation: Consultancy, Research Funding. Wang:Celgene Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal