
Background: Relapse remains the most common cause of treatment failure after intensive induction and consolidation (CONS) therapy in older adults with AML. We therefore performed a prospective randomized phase II study to determine the safety and impact on DFS (relapse or death) and OS of DAC maintenance using an abbreviated 3-day schedule administered every 4 weeks for 1 year (per Lubbert et al, Haematologica 97:393, 2012) vs. Observation (OBS) after intensive AML therapy, conducted in the large multi-center E-A E2906 Phase III trial in patients (pts) age ≥60 yrs.
Methods: The design and primary clinical results for E2906 (n=727) have been reported previously (Foran et al, ASH #217a, 2015), demonstrating superior OS following 'Standard' 7&3 (Daunorubicin 60mg/m2) induction and intermediate dose Ara-C consolidation (CONS) vs. single agent Clofarabine (CLO, provided by SANOFI), despite similar CR/CRi (CR with incomplete CBC recovery) and induction mortality rates. All CR/CRi pts after induction (n=311) were assigned to 2 cycles CONS with either Ara-C (1.5g/m2 x 12 doses; 6 doses if age >/=70 yrs), or single agent CLO, based on induction randomization.
Ongoing CR/CRi after recovery from CONS was confirmed with restaging BM biopsy, and eligible pts offered participation in the 'Step 3' maintenance study, a 1:1 randomization (stratified by induction therapy, cytogenetic risk group, age <70 yrs) to either OBS vs. DAC (20mg/m2IV Days 1-3, q4 wks) for 1 year. DAC could be held for up to 4 weeks for delayed hematologic recovery, but dose reductions were not permitted per protocol, and DAC was not continued beyond 12 months. Patients in both arms were evaluated with CBC q4 weeks for 1 year, and surveillance BM biopsy every 3 months (or at the time of suspected clinical relapse) for 2 years.
'Step 3' was designed as a phase II pilot randomized study, with target accrual n=172, allowing 90% power to detect 36% reduction in DFS Hazard Ratio (HR) with DAC maintenance for 1 year (using protocol-specified 1-sided log-rank test significance level of 0.1), assuming 140 events & 2 years of follow-up from randomization. However, further accrual to E2906 was suspended in 2/2015 by independent DSMC due to superior OS observed with standard Arm, so that 'Step 3' completed only 70% of target accrual. P-values reported are two-sided by convention unless specified otherwise.
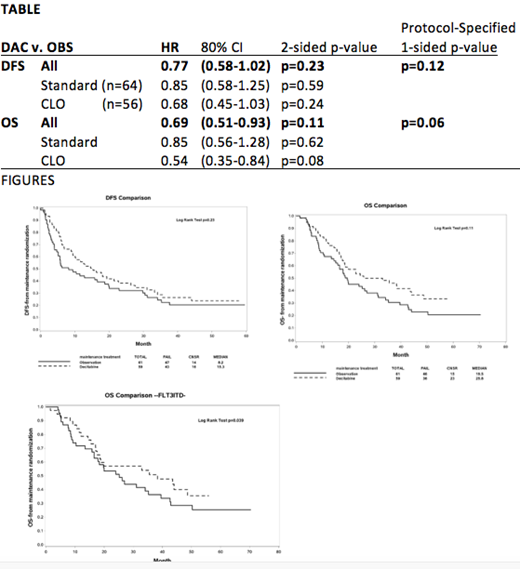
Results: 'Step 3' total accrual was n=120 (of 172 planned) (DAC 61, OBS 59), with median age 69 yrs (range 60-85), and groups were very well balanced for baseline clinical characteristics; most pts had Intermediate risk cytogenetics (74.2%) and ECOG performance status (PS)=0/1 (96%). The median number of DAC cycles received was 6 (range 0-13), and analysis was 'intention-to-treat'. The median follow-up is 49.8 months. There were 90 DFS events (47 OBS, 43 DAC), with 82 deaths (46 OBS, 36 DAC). Univariate DFS and OS are shown in Figure, with associated two-sided p-values; using protocol-prespecified one-sided significance level of 0.1, OS was significant (one-sided p=0.06) for DAC v. OBS, and there was a statistical trend favoring DFS (one-sided p=0.11), particularly for CLO pts (Table).
We also performed multivariate Cox model (after adjustment for ECOG PS, sex, secondary AML, and baseline hematologic parameters) which confirmed superior HR for DFS (one-sided p=0.10) & OS (one-sided p=0.07), per statistical design.
FLT3-ITD status is available for n=96 pts, and 84 were FLT3-ITD-negative (46 OBS, 38 DAC). Importantly, we observed a significant association of DAC maintenance with superior OS in the large FLT3-ITD-neg subgroup (p=0.039) (Figure).
DAC was generally well tolerated apart from grade 3 febrile neutropenia (9%) and reversible grade 4 cytopenias, with no grade 5 events.
Conclusions: In this randomized phase II study, DAC maintenance for 1 year after intensive AML therapy was associated with improved HR for OS and a trend for DFS, using protocol-specified statistical design. Furthermore there was a significant impact on OS for the FLT3-ITD-negative population. We acknowledge limitations based on incomplete accrual due to early termination of the parent E2906 study, and inherent to the phase 2 design of this E2906 endpoint, but results suggest an important impact of DAC maintenance on survival. These data strongly support a definitive phase III randomized study of DAC maintenance, particularly focused on the large intermediate risk FLT3-ITD-negative subgroup.
Foran:Agios: Honoraria, Research Funding. Claxton:Daiichi Sankyo Co. and Ambit Biosciences Corp, Astellas Pharma, Novartis Pharmaceuticals, Incyte Corporation, Cyclacel Pharmaceuticals, Inc, Celegene Corporation, Medimmune, Inc, Merck Sharp & Dohme Corp., Gilead Sciences, Inc.: Research Funding. Lazarus:Pluristem Therapeutics, Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Genentech: Speakers Bureau; Biosight: Membership on an entity's Board of Directors or advisory committees; Actinium Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Adaptive Biotechnologies: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; Teva Pharmaceuticals: Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Consultancy. Rowe:BioSight: Consultancy. Altman:Cancer Expert Now: Consultancy; Glycomimetics: Consultancy, Honoraria, Other: Data Safety and Monitoring Committee; prIME Oncology: Speakers Bureau; PeerView: Speakers Bureau; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Theradex: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; France Foundation: Speakers Bureau; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Biosight: Other: US Lead. Luger:Ariad: Research Funding; Agios: Honoraria; Biosight: Research Funding; Seattle Genetics: Research Funding; Pfizer: Honoraria; Onconova: Research Funding; Kura: Research Funding; Jazz: Honoraria; Genetech: Research Funding; Daichi Sankyo: Honoraria; Cyslacel: Research Funding; Celgene: Research Funding. Al-Kali:Astex Pharmaceuticals, Inc.: Research Funding. Zheng:Pfizer: Research Funding. Pratz:AbbVie: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Millenium/Takeda: Research Funding; Boston Biomedical: Consultancy; Astellas Pharma: Consultancy, Research Funding. Powell:Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding; Janssen: Research Funding; Novartis: Consultancy, Speakers Bureau; Rafael Pharmaceuticals: Consultancy, Research Funding. Tallman:Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; UpToDate: Patents & Royalties; Cellerant: Research Funding; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Use of maintenance Decitabine in AML
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal