Background: Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare, life-threatening autoimmune thrombotic microangiopathy caused by a deficiency in the activity of ADAMTS13 leading to the formation of ultra-large multimers of von Willebrand factor (vWF) and abnormal platelet adhesion in the microvasculature. aTTP requires prompt diagnosis and rapid initiation of treatment to limit the risk of negative or fatal outcomes. The clinical diagnosis of aTTP is based on thrombocytopenia and microangiopathic hemolytic anemia and is confirmed by ADAMTS13 <10%. However, the latter confirmation is not always rapidly available, and treatment is typically initiated based on the clinical diagnosis. The HERCULES study, in which patients were enrolled based on the clinical diagnosis of aTTP (ADAMTS13 confirmation was not part of the eligibility criteria) after receiving 1 prior session of therapeutic plasma exchange (TPE), demonstrated the efficacy and safety of caplacizumab in patients experiencing an acute aTTP episode (Scully et al. N Engl J Med 2019;380:335-346); caplacizumab targets the A1 domain of vWF, disrupting formation of microthrombi. The main safety finding in HERCULES was a mild bleeding risk. This analysis aimed to describe the safety of caplacizumab in patients enrolled in HERCULES for whom the diagnosis of aTTP was not confirmed based on documented severe ADAMTS13 deficiency.
Methods: In HERCULES, ADAMTS13 was measured at study baseline (following initial TPE), weekly following cessation of daily TPE during the treatment period, and twice during the follow-up period. Data from patients for whom the diagnosis of aTTP was not confirmed based on documented ADAMTS13 levels <10% were extracted and analyzed descriptively for efficacy and safety outcomes, with a focus on bleeding events.
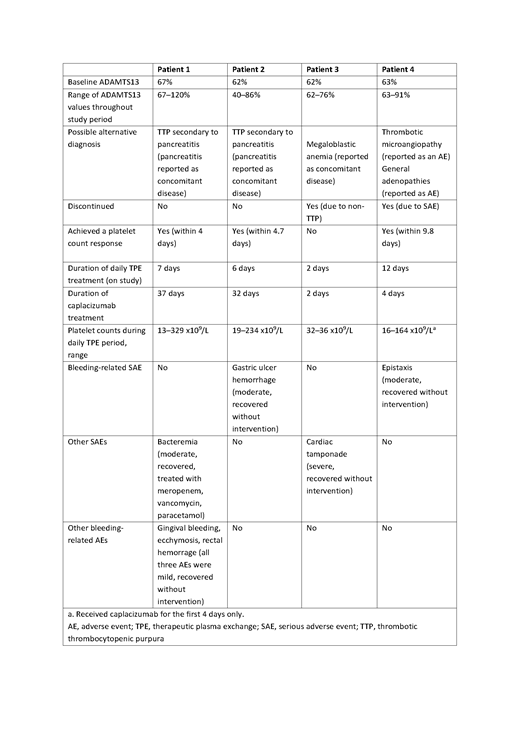
Results: Overall, 7 patients in the placebo group (9.6%) and 13 patients in the caplacizumab group (18.1%) had a baseline ADAMTS13 ≥10%; of these, 4 and 9 patients, respectively, had a prior medical history of aTTP and/or ADAMTS13 values <10% at other time points during the study. This left 3 patients in the placebo group and 4 patients in the caplacizumab group for whom the diagnosis of aTTP could not be confirmed based on subsequent ADAMTS13 values or prior medical history, suggesting a diagnosis other than aTTP. The baseline characteristics and outcomes for those patients treated with caplacizumab are summarized in Table 1. Baseline ADAMTS13 levels were >60% for all patients and remained well above 10% throughout the study period. Possible alternative diagnoses included pancreatitis-induced TTP in 2 patients. One patient was reported as having 'thrombotic microangiopathy' and discontinued study drug treatment after 4 days (but continued daily TPE). The fourth patient had a report of 'megaloblastic anemia' and 'general adenopathies' and was withdrawn from the study due to a 'non-TTP diagnosis' after 2 days. The patients who continued daily TPE achieved a platelet count of >150 x109/L. Two patients experienced a moderate bleeding-related serious adverse event (SAE), 1 case of 'gastric ulcer hemorrhage' (considered unlikely related to study drug and recovered without intervention) and 1 case of epistaxis that led to study drug discontinuation (considered possibly related to study drug and recovered without intervention). Other mild bleeding-related non-serious adverse events (AEs) were reported in 1 patient: gingival bleeding (possibly related), ecchymosis (possibly related), and rectal hemorrhage (not/unlikely related). All events recovered spontaneously without intervention. Two other non-bleeding related SAEs were reported in 2 patients, both considered unrelated to study drug: 1 case of bacteremia and 1 case of cardiac tamponade.
Conclusion: The experience of caplacizumab in patients with a suspected non-aTTP diagnosis to date is limited, and so no definite conclusion can be drawn. Bleeding-related AEs were reported in 3 of the 4 patients; however, the type, nature and manageability of these events appear similar to those reported in the other patients in the study.
Table 1. Baseline characteristics and outcomes during the study period for patients with baseline ADAMTS13 ≥10% treated with caplacizumab in the HERCULES study.
De La Rubia:Celgene Corporation: Consultancy; Janssen: Consultancy; AMGEN: Consultancy; Takeda: Consultancy; AbbVie: Consultancy. Peyvandi:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alnylam: Honoraria; Grifols: Honoraria; Kedrion: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Octapharma: Research Funding. Scully:Shire/Takeda: Consultancy; Novartis: Consultancy; Shire: Research Funding; Alexion: Consultancy; Ablynx/Sanofi: Consultancy. Cataland:Ablynx/Sanofi: Consultancy, Research Funding; Alexion: Consultancy, Research Funding. Coppo:Shire: Consultancy; Ablynx/Sanofi: Consultancy; Alexion: Consultancy. Kremer Hovinga:Shire: Consultancy, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology), Research Funding; Siemens: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Roche: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); CSL-Behring: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Ablynx/Sanofi: Consultancy, Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology). Knoebl:Novo-Nordisk: Consultancy, Research Funding; Ablynx/Sanofi: Consultancy; Shire/Takeda: Consultancy; CSL-Behring: Consultancy; Roche: Consultancy. Metjian:AblynxNV/Sanofi: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Pavenski:Alexion: Honoraria, Research Funding; Shire: Honoraria; Bioverativ: Research Funding; Octapharma: Research Funding; Ablynx: Honoraria, Research Funding. De Winter:Ablynx, a Sanofi company: Employment. de Passos Sousa:Sanofi: Employment. Callewaert:Sanofi (formerly employed by Ablynx, a Sanofi company): Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal