Introduction: Mature blood cells harbor a mixture of mutations inherited from ancestral hematopoietic stem cells (HSCs) and mutations accumulated after maturation. The landscape of these somatic mutations in normal blood is poorly mapped, with questions as simple as "how many mutations does a memory T cell accumulate throughout life?" remaining unanswered. This gap in our knowledge is particularly relevant for hematopoietic malignancy- while we know that lymphomas derive from lymphocytes of particular stages of differentiation, we do not know if the patterns we see are reflected in their normal counterparts.
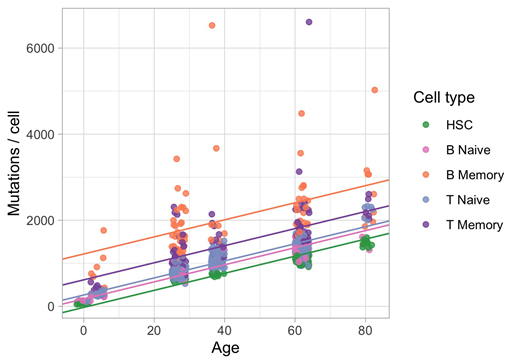
Results: In order to characterize normal somatic mutation in lymphocytes, we performed single-cell expansion and whole genome sequencing of over 600 T and B lymphocytes and 200 HSC and progenitor cells across 5 individuals (ages 0-85). All lymphocyte subsets show increased mutation burden with respect to HSCs across all classes of variants (Figure 1). Some of this increase can explained by lymphocyte-specific mutational processes, such as the activity of RAG, accounting for at least 20% of observed structural variants. We also find a striking variation in mutation burden within and between lymphocyte subsets. Microenvironment specific mutational processes dominate the observed differences. Examples of this include germinal center ("non-canonical AID") mutations in memory B cells and UV-like mutations in memory T cells (putatively skin resident cells). Naive B and T cells show a lack of variation in discrete mutational patterns relative to their memory counterparts, and have mutational profiles and mutation burdens more similar to that of HSCs. We also observe differences in the mutational patterns between B and T cells that are indicative of the increased divergence of T lymphocytes from the HSC pool. In general, the mutation burden we observe in normal lymphocytes approach those seen in lymphoma.
Conclusions: Our work highlights the substantial genetic diversity in normal lymphocytes, with some cells accumulating thousands of mutations on top of those inherited from the HSC compartment. These mutations can be used to describe the life history of each individual lymphocyte including their exposure to specific microenvironments. Our findings shed light on the biology of these cells and will help differentiate between normal and disease processes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal