Introduction: Fetal hemoglobin (HbF) expression is a major modulator of sickle cell disease (SCD) severity by decreasing the HbS polymerization. However, the distribution of HbF in red blood cells (RBC) is heterogeneous in SCD patients. In the hypothesis of an HbF "threshold" in RBCs for inhibiting the HbS polymerization, accurate measurement of the HbF content in each red blood cell (HbF/RBC) is mandatory. To this purpose we developed a new and accurate method allowing the direct measurement of HbF content per RBC. Thanks to it, as a proof of concept, we analyzed HbF distribution and content in RBC from SCD patients before and after 6 months of treatment by hydroxyurea (HU). To determine if a threshold of HbF/RBC could modulate SCD, we analyzed the associations between the %RBC reaching different thresholds of HbF (in picograms), and biological parameters and the incidence of severe VOC.
Methods: 14 SCD (βS/βS or βS/β0) patients were included to study HbF distribution during HU for a period of 6 months. RBCs were collected during each outpatient visit (Week 0, Week 2, Week 4, Week 12, and ≥ Week 24). HbF content was measured in RBCs using an anti-Human-HbF antibody by flow cytometry. Normalized RBC fluorescence intensity was then converted in picograms of HbF/RBC by using the linear association between mean HbF content and mean RBC fluorescence obtained from subjects presenting homogeneous HbF distribution (patients with hereditary persistence of HbF (HPFH), or β-Thalassemia or δβ-Thalassemia). Quantitative analysis were performed before and during HU treatment to characterize the response by comparing percentages of RBC classes based on different ranges of HbF/RBC during HU treatment. We therefore analyzed the associations between HbF/RBC thresholds (%RBC containing at least 2, 4, 6, 8, 10 or 20 pg HbF) and biological parameters before and ≥ 6 months of HU treatment. Finally HbF/RBC thresholds at Week 0 were compared to the incidence of hospitalized VOC within 3 years before W0, and HbF/RBC thresholds at Week 24 were compared to the incidence of hospitalized VOC within 3 years after W24 at a stable dose.
Results: After 6 months of HU, mean %HbF, assessed by HPLC, raised from 6.16% (±3.5) to 15.2% (±8.7) (mean ± standard deviation). Quantitative analysis of HbF/RBC revealed a statistically significant decrease between D0 and ≥M6 of 24% of RBCs containing less than 2 pg (p = 0.0015) and a 2-fold increase of RBCs containing between 2 and 4 pg (p = 0.0025) (Friedman test).
For biological parameters we observed an increase in mean %HbF, MCV and MCH and a decrease in RBC count significantly associated (p<0.001 - Spearman test) with %RBC containing ≥ 2 pg of HbF.
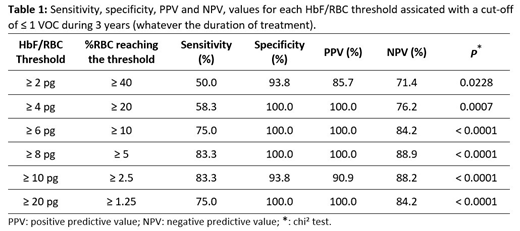
The incidence of VOC within 3 years after HU treatment was not statistically significant than during the 3 years before (p = 0.4414 - Wilcoxon test). VOC incidence under treatment decreased in 6/14 patients, did not change in 4/14 and increased in 4/14. The incidence of VOC over 3 years was not associated with the %HbF assessed by HPCL (r = -0.0358; p = 0.8564 - Spearman test). We observed a statistically significant correlation between the incidence of VOC over 3 years and the HbF threshold of 4 pg (r = -0.5068; p = 0.0059). We therefore determined the percentage of RBCs by thresholds of HbF, associated to ≤ 1 VOC over 3 years (Table 1). For example, if more than 20% RBCs have ≥ 4 pg of HbF, we calculated a sensitivity and a specificity of 58.3% and 100% respectively, and a positive and a negative predictive value of 100.0% and 76.2% respectively, to have ≤ 1 VOC over 3 years.
Conclusion: Our results strengthen the hypothesis that the percentage of RBC above a threshold of HbF is the important parameter to measure. These results need to be replicated in a larger cohort but they open up interesting prospects for analysis of new therapeutic efficacy, including gene therapy and HbF inducers.
Galactéros:Addmedica: Membership on an entity's Board of Directors or advisory committees. Bartolucci:Agios: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; HEMANEXT: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; AddMedica: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal