Background: Sickle cell disease (SCD) is the most common single-gene disorder in African Americans and can lead to complications, including acute pain and chronic organ damage. Approximately 40% of men with SCD experience priapism, a clinical disorder characterized by prolonged, painful penile erection in the absence of sexual stimulation. Vaso-occlusion-induced ischemia is generally thought to account for SCD-associated priapism. Crizanlizumab, a humanized monoclonal antibody that binds P-selectin and blocks interaction with its ligands (including leukocyte PSGL-1). Crizanlizumab significantly decreased vaso-occlusive crises (VOCs) leading to healthcare visit vs placebo, and was well tolerated in SUSTAIN, a phase 2 study in adults with SCD. This SPARTAN study aims to evaluate the clinical efficacy of intravenous (IV) crizanlizumab 5 mg/kg in reducing priapic events in patients with SCD and a history of priapism utilizing a modern electronic reporting system. Electronic reporting tools have advantages over the paper format in regards to accuracy of and compliance in recording PROs (Stone et al, 2003). The study design and details of PRO collection methods are presented here.
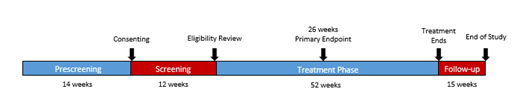
Study Design and Methods: This is a Phase 2, multicenter, open-label, single arm study of crizanlizumab in male patients aged ≥16 years with SCD-related priapism. The study will consist of 14 weeks prescreening, 12 weeks screening, and 52 weeks of treatment. The primary endpoint is percent reduction from baseline of priapic events frequency (unwanted/painful erection lasting >60 minutes) by 26 weeks. It is expected that crizanlizumab treatment reduces priapic events by ≥ 25% in SCD patients with priapism by 26 weeks. Demonstration of significant percent reduction from baseline in priapic events will be evaluated statistically.
Priapic events will be self-reported in real time via a restricted and secure study-issued smart watch and smart phone with a study-specific digital application (app). The patient records the start of a priapic event using a button on the pin code-protected app, which triggers a report in a database, creates a date/time stamp, and records the patient's identification. The patient is then prompted to complete an event reporting survey in one of three ways: using the app on the watch/phone, by a personal call with an operator, or by paper diary. Event reporting surveys will record priapic event occurrences, time and length of events, how the event was relieved, if there was a trigger, and the patient-reported pain at its worst during the event (scale of 0-10). Paper diaries are available as a back-up.
Secondary endpoints include safety, as well as the following outcomes at 26 and 52 weeks: rate of priapic events, percent reduction in ≥4-hour erections requiring an ER visit, rate of VOC at 26 and 52 weeks, rate of uncomplicated VOC events, and rate of complicated crises. Approximately 56 patients are planned to be enrolled across 28 sites. Eligible patients must be ≥ 16 years old, have ≥4 events during prescreening, ≥3 during screening with 1 event occurring within 4 weeks prior to first treatment. Patients will be treated with IV crizanlizumab 5 mg/kg on the first day of Week 1, Week 3 (loading dose), Week 7, and then every 4 weeks until final treatment at Week 51. Primary analysis will be conducted after patients receive 26 weeks of treatment. Mandatory safety follow-ups will be conducted until 15 weeks after last dose. Patient feedback was considered and incorporated into the trial design.
Results: This Phase 2 study (ClinicalTrials.gov Identifier: NCT03938454) has been successfully designed and approved by the institutional review board. Trial is ongoing.
Conclusions: This study, which incorporates electronic PRO collection methods, has been designed to address the unmet treatment need in male patients >16 years old with SCD-related priapism.
Figure 1. SPARTAN Study Design
Darbari:Novartis: Membership on an entity's Board of Directors or advisory committees; Hilton Publishing: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Other: one day advisory board meeting . Paulose:Novartis Pharmaceuticals Corporation: Employment. Laine:Novartis: Employment. Purkayastha:Novartis Pharmaceuticals: Employment. Kato:Novartis, Global Blood Therapeutics: Consultancy, Research Funding; Bayer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal