TO THE EDITOR:
In vertebrates, hematopoietic stem/progenitor cells (HSPCs) first arise from the hemogenic endothelium in the aorta-gonad-mesonephros (AGM) region of the developing embryo1-3 and subsequently migrate to secondary niches where they expand in number and differentiate.4,5 The cellular mechanism or mechanisms allowing HSPC migration and hematopoietic organ colonization remain poorly understood. Travnickova et al recently reported that primitive macrophages dynamically interact with HSPCs in the AGM to allow HSPCs mobilization and colonization of hematopoietic organs for the establishment of definitive hematopoiesis.6 Using a distinct approach, we present data that challenge these conclusions, as we found that primitive macrophages are unessential for HSPC mobilization and subsequent definitive hematopoiesis.
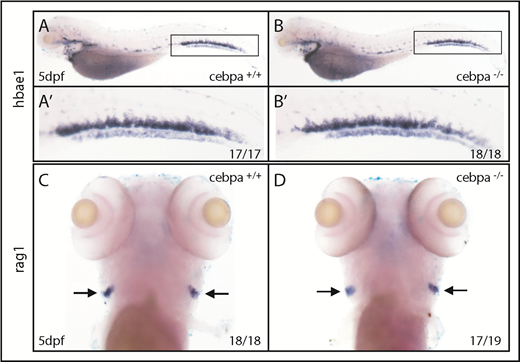
Travnickova et al chemically or genetically depleted primitive macrophages in zebrafish by liposome-encapsulated clodronate injection or by using inducible nitroreductase genetic depletion. They observed an accumulation of HSPCs in the AGM and a significant decrease in the colonization of the caudal hematopoietic tissue (CHT) or thymus. They concluded that HSPC mobilization and colonization require primitive macrophages. To examine whether macrophages indeed play a crucial role in HSPC mobilization and colonization, we used macrophage-deficient zebrafish embryos. Cebpa is a basic leucine zipper transcription factor that plays key roles in the regulation of myelopoiesis.7 We previously generated a cebpa mutant (cebparj31) using TALENs technology.8 These cebpa-deficient embryos lack primitive macrophages, as detected by whole-mount messenger RNA in situ hybridization (WISH) of mfap4 and mpeg1 (macrophage lineage markers; Figure 1A-D; supplemental Figure 1A-J, available on the Blood Web site) or neutral red staining, which preferentially labels macrophages in living embryos9 (supplemental Figure 1K-L). Similar macrophage depletion was also observed in another cebpa-deficient zebrafish generated by an N-ethyl-N-nitrosourea–induced forward genetic screening.10 Unexpectedly, when we examined expression of cmyb, a conserved HSPC marker, we did not observe abnormal HSPCs accumulation in the AGM nor a significant decrease in HSPCs in the CHT of macrophage-deficient cebparj31 mutant when compared with macrophage-sufficient siblings (Figure 1E-H). To further explore this issue, we outcrossed cebparj31 mutant with Tg(cd41:GFP) fish, where HSPCs are marked by the GFPlow subset.11,12 Again, the results showed that there was no significant difference in the number of HSPC/cd41:GFPlow+ cells between macrophage-deficient cebparj31 mutant and macrophage-sufficient siblings (Figure 1I-L). Taken together, these data suggest, in 2 independent settings, that macrophage absence has little effect on HSPC mobilization and colonization. Next, to determine whether HSPCs could differentiate into committed lineages when primitive macrophages are absent, we examined lineage-specific markers in macrophage-deficient cebparj31 mutant. Expression of erythroid lineage marker hbae1 or lymphoid lineage marker rag1 was relatively normal when compared with control siblings (Figure 2A-D), suggesting that the differentiation potential of HSPCs was not affected by absence of primitive macrophages.
Depletion of primitive macrophages does not affect HSPC mobilization and colonization of the CHT. (A-D) WISH of mfap4 shows macrophages are absent in cebparj31 mutant compared with control sibling. White arrows indicate mfap4-positive cells in the yolk sac. (E-H) WISH of cmyb shows macrophage-deficient cebparj31 mutants have normal levels of HSPC in the AGM and the CHT, respectively. (E'-H') Magnified images of corresponding boxed regions from panels E-H, respectively. (I-J) Fluorescent images of the CHT region of cebparj31/Tg(cd41:GFP) embryo at 72 hpf. (K) ImageJ analysis revealed no significant difference in the number of cd41:GFP+ cells in the CHT at 72 hpf. Two-tailed Student t test. (L) Flow cytometry analysis showed no significant changes in the relative number of HSPC/cd41:GFPlow+ cells between cebparj31 mutants and control siblings (0.756% ± 0.237% vs 0.599% ± 0.041%, P > .05, 2-tailed Student t test). The data are representative of 3 independent experiments. FSC-H, forward scatter height; GFP-A, green fluorescent protein area; hpf, hours postfertilization; n.s., not significant.
Depletion of primitive macrophages does not affect HSPC mobilization and colonization of the CHT. (A-D) WISH of mfap4 shows macrophages are absent in cebparj31 mutant compared with control sibling. White arrows indicate mfap4-positive cells in the yolk sac. (E-H) WISH of cmyb shows macrophage-deficient cebparj31 mutants have normal levels of HSPC in the AGM and the CHT, respectively. (E'-H') Magnified images of corresponding boxed regions from panels E-H, respectively. (I-J) Fluorescent images of the CHT region of cebparj31/Tg(cd41:GFP) embryo at 72 hpf. (K) ImageJ analysis revealed no significant difference in the number of cd41:GFP+ cells in the CHT at 72 hpf. Two-tailed Student t test. (L) Flow cytometry analysis showed no significant changes in the relative number of HSPC/cd41:GFPlow+ cells between cebparj31 mutants and control siblings (0.756% ± 0.237% vs 0.599% ± 0.041%, P > .05, 2-tailed Student t test). The data are representative of 3 independent experiments. FSC-H, forward scatter height; GFP-A, green fluorescent protein area; hpf, hours postfertilization; n.s., not significant.
Depletion of primitive macrophages has little effect on definitive hematopoiesis. (A-B) WISH of hbae1 shows erythropoiesis is not affected in cebparj31 mutant compared with control sibling. (A′-B′) Magnified images of corresponding boxed regions from panels A and B. (C-D) WISH of rag1 shows lymphopoiesis is relatively normal in cebparj31 mutant compared with control sibling. dpf, days postfertilization.
Depletion of primitive macrophages has little effect on definitive hematopoiesis. (A-B) WISH of hbae1 shows erythropoiesis is not affected in cebparj31 mutant compared with control sibling. (A′-B′) Magnified images of corresponding boxed regions from panels A and B. (C-D) WISH of rag1 shows lymphopoiesis is relatively normal in cebparj31 mutant compared with control sibling. dpf, days postfertilization.
The main difference between the 2 studies is that Travnickova et al used chemical methods to deplete macrophages (ie, clodronate liposome injection), whereas we used a genetic approach. Clodronate liposome eliminates macrophages by initiating their apoptosis so that the inflammatory factors released from apoptotic macrophages might affect HSPC development.13 Moreover, the injected clodronate liposomes may aggregate in the vessel and perturb blood circulation, which was shown to play a key role in HSPC development and mobilization.14 Critically, when we injected clodronate liposome into the caudal vein at 25 hpf, as was done by Travnickova et al,6 we found that the number of HSPCs was sharply decreased in clodronate liposome–injected embryos, even in macrophage-deficient cebparj31 mutants (supplemental Figure 2), indicating that the reduced HSPCs triggered by clodronate liposome is macrophage independent.
Travnickova et al proposed that macrophages control HSPC mobilization and colonization of hematopoietic organs via secretion of matrix metalloproteinases (Mmps) and extracellular matrix (ECM) remodeling. Mmp9, primarily expressed by macrophages, would play a key role in ECM degradation around HSPCs in the AGM region, allowing HSPC mobilization and migration.6 However, Mmps secreted by other cell types might also contribute to HSPC mobilization, intravasation, and hematopoietic organ colonization. For example, Theodore et al recently reported that vascular-associated Mmp2, but not Mmp9, facilitates HSPC budding and migration from the AGM to the CHT via ECM digestion.15 Moreover, we could observe some primitive neutrophils in the intermediate cell mass region in cebparj31 mutants at 22 hpf, although they were severely impaired from 30 hpf onwards (supplemental Figure 3A-J). These earlier neutrophils producing tumor necrosis factor-α might also facilitate HSPC emergence as proposed by previous study.16
In summary, our data suggest that primitive macrophages are not required for HSPC mobilization and definitive hematopoiesis during embryogenesis in zebrafish. More research is needed before definitive conclusions can be made on cellular and molecular mechanisms controlling HSPC mobilization and hematopoietic organ colonization.
Zebrafish maintenance and staging were performed as described previously.8 The Tg(cd41:GFP) and cebparj31 mutants were used in this study. The zebrafish facility and study were approved by the Institutional Review Board of the Institute of Health Sciences, Shanghai Institutes of Biological Sciences, and Chinese Academy of Sciences (Shanghai, China), and the methods were carried out in accordance with the approved guidelines.
Digoxigenin-labeled antisense RNA probes were transcribed from linearized constructs using T3 or T7 polymerase (Roche). WISH was performed as previously described.8 The probes were detected using alkaline phosphatase-coupled anti-digoxigenin Fab fragment antibody (Roche) with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium staining (Vector Laboratories).
Live embryos were incubated in 2.5 μg/mL neutral red (in embryo medium) at 28.5°C in the dark for 5 to 8 hours as previously described.9 The 4% formaldehyde-fixed embryos were incubated in Sudan black (Sigma-Aldrich) solution for 20 minutes, washed by 70% ethanol, and then rehydrated to PBS and 0.1% Tween 20.
cebparj31/Tg(cd41:GFP) embryos at 72 hpf were dissected, and the trunk regions were digested with 0.25% trypsin (Thermo Fisher Scientific) for 15 minutes at 28.5°C. Single-cell suspension was obtained by passing through a 40-μm nylon mesh filter. The flow cytometric data were collected on a BD LSRFortessa flow cytometer and analyzed using FlowJo software.
Liposome-encapsulated clodronate was injected into the caudal vein at 25 hpf as previously described.6
The online version of this article contains a data supplement.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (31371479).
Authorship
Contribution: H.Y. designed the research, performed experiments, analyzed data, and wrote the manuscript; S.G. and H.C. performed the experiments; and X.L., J. Zhou, H.d.T., and J. Zhu provided suggestions on experimental design and data presentation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hao Yuan, Rui-Jin Hospital, Room 1108, Building 11, 197 Ruijin Rd II, Shanghai 200025, China; e-mail: hyuan@sibs.ac.cn; and Jun Zhu, Rui-Jin Hospital, Room 1108, Building 11, 197 Ruijin Rd II, Shanghai 200025, China; e-mail: zhuj1966@yahoo.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal