Key Points
Compared with rituximab only, a short rituximab-lenalidomide regimen improved CR rate and PFS in untreated symptomatic follicular lymphomas.
Excellent OS in both arms suggests that chemotherapy-free strategies should be further explored in follicular lymphoma.
Abstract
The SAKK 35/10 phase 2 trial, developed by the Swiss Group for Clinical Cancer Research and the Nordic Lymphoma Group, compared the activity of rituximab vs rituximab plus lenalidomide in untreated follicular lymphoma patients in need of systemic therapy. Patients were randomized to rituximab (375 mg/m2 IV on day 1 of weeks 1-4 and repeated during weeks 12-15 in responding patients) or rituximab (same schedule) in combination with lenalidomide (15 mg orally daily for 18 weeks). Primary end point was complete response (CR)/unconfirmed CR (CRu) rate at 6 months. In total, 77 patients were allocated to rituximab monotherapy and 77 to the combination (47% poor-risk Follicular Lymphoma International Prognostic Index score in each arm). A significantly higher CR/CRu rate at 6 months was documented in the combination arm by the investigators (36%; 95% confidence interval [CI], 26%-48% vs 25%; 95% CI, 16%-36%) and confirmed by an independent response review of computed tomography scans only (61%; 95% CI, 49%-72% vs 36%; 95% CI, 26%-48%). After a median follow-up of 4 years, significantly higher 30-month CR/CRu rates and longer progression-free survival (PFS) and time to next treatment (TTNT) were observed for the combination. Overall survival (OS) rates were similar in both arms (≥90%). Toxicity grade ≥3 was more common in the combination arm (56% vs 22% of patients), mainly represented by neutropenia (23% vs 7%). Addition of lenalidomide to rituximab significantly improved CR/CRu rates, PFS, and TTNT, with expected higher, but manageable toxicity. The excellent OS in both arms suggests that chemotherapy-free strategies should be further explored. This trial was registered at www.clinicaltrials.gov as #NCT01307605.
Introduction
The outcome of patients with follicular lymphoma (FL) has improved continuously during the last 2 decades,1-5 mainly because of introduction and development and of immunochemotherapeutic approaches based on rituximab (R).6-8 The expected median survival currently approaches or even exceeds 20 years, particularly in younger patients.3,9 However, the disease is clinically heterogeneous. Some patients live for decades without the need for treatment, whereas in others, the disease is rapidly progressive.10,11
For asymptomatic patients with advanced-stage disease but low tumor burden, a watchful waiting policy has long remained a widely accepted approach.12 This strategy has been challenged by randomized studies showing that R monotherapy in these patients results in delayed time to new treatment with minimum toxicity, although without a survival benefit.13,14
For symptomatic patients with advanced disease and in need of treatment, the combination of R and chemotherapy, mostly followed by R maintenance, has become standard treatment in many countries.15-18 Several new targeted drugs have also shown clinical efficacy and a favorable toxicity profile in FL, holding promise for a future with safe and effective chemotherapy-free treatments.
The long-term results of clinical studies developed and conducted by the Swiss Group for Clinical Cancer Research (SAKK) and the Nordic Lymphoma Group (NLG) have demonstrated that R monotherapy can produce durable remissions in a sizeable subset of FL patients, with overall survival (OS) similar to that achieved with immunochemotherapy but with less toxicity, providing a rationale for further development of chemotherapy-free treatment strategies.4,19-24 Because the expected survival of FL patients is continuously improving, the safety of long-term treatment is becoming increasingly important, and there is a strong interest in developing therapeutic alternatives that may limit or delay cytotoxic exposure with its potentially serious late toxicity.25
In the exploration of chemotherapy-free therapies, lenalidomide (L) was found to boost natural killer cell– and monocyte-mediated antibody-dependent cellular cytotoxicity, thereby enhancing the activity of R against CD20+ tumor cells in vivo.26-28 Moreover, the synergism was supported by promising results of early clinical trials.29-31 Notably, responses occurred irrespective of tumor bulk, stage, or symptoms, which suggests that chemotherapy-free approaches may be applied to both low– and high–tumor burden patients.
We report here the results of the SAKK 35/10 phase 2 trial, developed and conducted by the SAKK in cooperation with the NLG to compare the activity of R plus L (RL) vs single-agent R as first-line therapy for symptomatic FL patients.
Patients and methods
Study design and treatment
Patients with untreated FL, grade 1 to 3A with CD20 expression by immunohistochemistry, stage III or IV or stage II and not suitable for radiotherapy, were eligible if in need of systemic therapy, defined by the presence of ≥1 of the following conditions: symptomatic enlarged lymph nodes or spleen or other lymphoma manifestations, bulky disease (longest diameter ≥6 cm), clinically significant progression over ≥6 months of any tumor lesion, B symptoms, hemoglobin <100 g/L or platelets <100 × 109/L, or any clinically significant progressive decrease in hemoglobin or platelet count as a result of lymphoma.
Diagnosis of FL confirmed by central pathology review, with no sign of histological transformation, and presence of measurable disease were mandatory. Inclusion criteria included World Health Organization performance status ≤2 and adequate cardiac function. Adequate blood counts and hepatic function were also required, unless impairment was due to FL. Patients with impaired renal function (creatinine clearance <30 mL/min) were not eligible, nor were those with prior or concomitant additional malignancies within 5 years (with the exception of adequately treated cervical carcinoma in situ or localized nonmelanoma skin cancer) or those with an underlying medical condition that could impair their ability to participate in the trial. Pregnant and breastfeeding women were excluded, and all patients (men and women) had to agree to follow the pregnancy prevention measures required for L. Patients in need of an urgent response (eg, because of existing or imminent organ compression) were also excluded. Details on eligibility criteria are provided in the supplemental data.
Eligible patients were randomized centrally to either R monotherapy (375 mg/m2 IV on day 1 of weeks 1-4 and repeated during weeks 12-15 in responding patients) or to R (administered at the same schedule) in combination with L (15 mg orally daily, starting 14 days before the first R administration and continuously administered until 14 days after the last, up to a total 18 weeks). Random assignment to treatment arms was stratified for histological grade (1-2 vs 3A), bulky disease (<6 vs ≥6 cm), Follicular Lymphoma International Prognostic Index (FLIPI) score (1-2 vs ≥3), and center using the minimization method. The trial was not masked.
The R schedule was based on the results of previous FL trials by the NLG.20,22 No adaptation of R dose was allowed, whereas the L dose was adapted to renal function and adverse events, respectively. For patients with moderate renal insufficiency (creatinine clearance <60 but ≥30 mL/min), the starting dose of L was 5 mg. R could be delayed and L dose delayed or reduced in the case of severe (grade 3 or 4) neutropenia and/or thrombocytopenia according to protocol (supplemental Table 2). If either L or R had to be permanently suspended, the treatment continued as single-agent therapy. Trial treatment was discontinued in patients with less than minor response (MR; defined as ≥25% reduction in the sum of the product of tumor diameters) at first restaging of week 10 (±1 week), and these patients were treated at the discretion of the local investigator.
The trial was approved by the local and/or national ethics committees as appropriate, and conducted according to the Declaration of Helsinki. All patients provided written consent.
Patient evaluation
Initial staging included physical examination, standard laboratory assessments, computed tomography (CT) scans of the neck, chest, abdomen, and pelvis, and bone marrow biopsy. CT scans were performed within 6 weeks before randomization, at week 10 (±1 week), and at week 23 (±1 week) and were independently reviewed by 2 expert radiologists in a planned independent response review (IRR) of CT scans only. If bone marrow was involved at baseline, a biopsy was required at restaging. Additional posttreatment CT scans were mandatory at 30 months and 5 years after randomization for patients without earlier documented progression/relapse.
Follow-up assessments included routine blood counts, β2 microglobulin and lactate dehydrogenase evaluation every 3 months, physical examination every 6 months, and chest X-ray and abdominal ultrasound (or CT scan/magnetic resonance imaging if deemed necessary) every 12 months. Response was evaluated according to the National Cancer Institute International Workshop criteria.32
Outcome measures and statistical methods
The primary end point was the complete response (CR)/unconfirmed CR (CRu) rate at week 23 (±2 weeks) assessed by the investigators (with central revision of reported measurements for completeness and plausibility). Secondary end points were overall response rate, CR/CRu rate at 30 months (CR30),33 progression-free survival (PFS; calculated from randomization to disease progression or death), duration of CR/CRu (DOR), time to next treatment (TTNT), OS, and treatment toxicity. Time-dependent end points were defined according to the revised National Cancer Institute criteria.34 PFS and DOR were estimated using the investigator assessment. Any assessment within a window of 27 to 33 months after randomization was to be considered the 30-month response assessment for determining the CR30 status.
Sample size (n = 152) was calculated to detect a 20% increase in the CR/CRu rate, with 90% power and type 1 error of 0.10 (1 sided). The CR/CRu rate and the corresponding two-sided Clopper-Pearson 95% confidence interval (CI) were calculated for each treatment arm. A 1-sided z test for proportions was used to compare the 2 arms. The type 1 and 2 error rates and the 1-sided test were chosen according to recommended policies for the optimization of phase 2 study design.35,36 Survival functions were estimated by the Kaplan-Meier method and treatment arms compared by the log-rank test. Cox proportional hazards models were also used for the estimation of hazard ratios (HRs). All randomly assigned patients were included in the analyses on an intention-to-treat (ITT) basis. Sensitivity analyses, using response assessment from IRR, were conducted based on the per-protocol set (subset of the ITT population who fulfilled major entry criteria, received effective amount of trial drug, and had tumor assessments at week 10, week 23, or progression/relapse). Effects of stratification factors were explored by logistic regression for CR/CRu at week 23 and Cox proportional hazards models for time-to-event end points.
Results
Patient characteristics and treatment
Between April 2011 and October 2013, 154 patients were randomized from 17 centers in Switzerland, 5 in Sweden, 3 in Norway, 2 in Italy, and 1 each in Denmark and Finland. Seventy-seven patients were allocated to R monotherapy (R arm) and 77 to RL (RL arm). Figure 1 shows the patients’ flow through the trial, and their baseline characteristics are summarized in Table 1. All patients were in need of therapy (indications for treatment start are provided in the supplemental data).
CONSORT diagram of trial profile and patient flow. PD, progressive disease; SD, stable disease.
CONSORT diagram of trial profile and patient flow. PD, progressive disease; SD, stable disease.
Patient characteristics
| Characteristic . | All Patients (N = 154) . | R (n = 77) . | RL (n = 77) . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Median (range) age, y | 62 (26-85) | 63 (29-85) | 61 (26-80) | |||
| ≥60 | 91 | 59 | 49 | 64 | 42 | 55 |
| ≥70 | 31 | 20 | 13 | 17 | 18 | 23 |
| Sex | ||||||
| Male | 72 | 47 | 37 | 48 | 35 | 45 |
| Female | 82 | 53 | 40 | 52 | 42 | 55 |
| Ann Arbor stage | ||||||
| II | 19 | 12 | 8 | 10 | 11 | 14 |
| III | 58 | 38 | 29 | 38 | 29 | 38 |
| IV | 77 | 50 | 40 | 52 | 37 | 48 |
| Performance status | ||||||
| 0 | 112 | 73 | 53 | 69 | 59 | 77 |
| 1 | 42 | 27 | 24 | 31 | 18 | 23 |
| B symptoms | ||||||
| Absent | 118 | 77 | 57 | 74 | 61 | 79 |
| Present | 36 | 23 | 20 | 26 | 16 | 21 |
| LDH* | ||||||
| Normal | 119 | 77 | 59 | 77 | 60 | 78 |
| Elevated | 32 | 21 | 17 | 22 | 15 | 19 |
| β2MG† | ||||||
| Normal | 83 | 54 | 40 | 52 | 43 | 56 |
| Elevated | 60 | 39 | 30 | 39 | 30 | 39 |
| Bulky disease, cm | ||||||
| <6 | 91 | 59 | 46 | 60 | 45 | 58 |
| ≥6 | 63 | 41 | 31 | 40 | 32 | 42 |
| Risk by FLIPI score | ||||||
| Low | 36 | 23 | 15 | 19 | 21 | 27 |
| Intermediate | 46 | 30 | 26 | 34 | 20 | 26 |
| High | 72 | 47 | 36 | 47 | 36 | 47 |
| Histologic grade | ||||||
| 1 | 38 | 25 | 18 | 23 | 20 | 26 |
| 2 | 91 | 59 | 46 | 60 | 45 | 58 |
| 3A | 25 | 16 | 13 | 17 | 12 | 16 |
| Characteristic . | All Patients (N = 154) . | R (n = 77) . | RL (n = 77) . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Median (range) age, y | 62 (26-85) | 63 (29-85) | 61 (26-80) | |||
| ≥60 | 91 | 59 | 49 | 64 | 42 | 55 |
| ≥70 | 31 | 20 | 13 | 17 | 18 | 23 |
| Sex | ||||||
| Male | 72 | 47 | 37 | 48 | 35 | 45 |
| Female | 82 | 53 | 40 | 52 | 42 | 55 |
| Ann Arbor stage | ||||||
| II | 19 | 12 | 8 | 10 | 11 | 14 |
| III | 58 | 38 | 29 | 38 | 29 | 38 |
| IV | 77 | 50 | 40 | 52 | 37 | 48 |
| Performance status | ||||||
| 0 | 112 | 73 | 53 | 69 | 59 | 77 |
| 1 | 42 | 27 | 24 | 31 | 18 | 23 |
| B symptoms | ||||||
| Absent | 118 | 77 | 57 | 74 | 61 | 79 |
| Present | 36 | 23 | 20 | 26 | 16 | 21 |
| LDH* | ||||||
| Normal | 119 | 77 | 59 | 77 | 60 | 78 |
| Elevated | 32 | 21 | 17 | 22 | 15 | 19 |
| β2MG† | ||||||
| Normal | 83 | 54 | 40 | 52 | 43 | 56 |
| Elevated | 60 | 39 | 30 | 39 | 30 | 39 |
| Bulky disease, cm | ||||||
| <6 | 91 | 59 | 46 | 60 | 45 | 58 |
| ≥6 | 63 | 41 | 31 | 40 | 32 | 42 |
| Risk by FLIPI score | ||||||
| Low | 36 | 23 | 15 | 19 | 21 | 27 |
| Intermediate | 46 | 30 | 26 | 34 | 20 | 26 |
| High | 72 | 47 | 36 | 47 | 36 | 47 |
| Histologic grade | ||||||
| 1 | 38 | 25 | 18 | 23 | 20 | 26 |
| 2 | 91 | 59 | 46 | 60 | 45 | 58 |
| 3A | 25 | 16 | 13 | 17 | 12 | 16 |
β2MG, β2 microglobulin; FLIPI, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase.
LDH values were missing in 4 patients.
β2MG values were missing in 11 patients.
Eight cycles of R were completed as planned by 71% of patients in the R arm and 91% in the RL arm, where 58% of patients received ≥90% of the planned dose of L and 29% had ≥1 dose-level reduction. A subsequent dose escalation was possible in 7 of 10 patients who began treatment at a lower dose level according to their baseline creatinine clearance.
Treatment was discontinued according to the protocol because of insufficient response at week 10 in 16 (21%) of 76 patients treated in the R arm and in 3 (4%) of 77 patients in the RL arm. In total, 14 patients stopped because toxicity: 1 in the R arm and 13 (11 patients stopped L only) in the RL arm (Figure 1).
Treatment outcomes
Results on treatment outcome are based on data from the latest follow-up in October 2017, with a median follow-up of the ITT population of 4 years (interquartile range, 3.3-4.7).
The primary end point analysis at week 23 showed a higher CR/CRu rate in patients treated with RL in comparison with those receiving R (Table 2). This difference was observed in both the ITT population (CR/CRu rate, 36%; 95% CI, 26%-48% vs 25%; 95% CI, 16%-36%, respectively; P = .056) and the per-protocol population (n = 124; CR/CRu rate, 40%; 95% CI, 28%-54% vs 27%; 95% CI, 17%-39%, respectively; P = .055) and is statistically significant according to the predefined 1-sided type 1 error of 0.10. The impact of the activity of the RL combination also remained higher (CR/Cru rate, 33%; 95% CI, 13%-59%) compared with R monotherapy (CR/Cru rate, 8%; 95% CI, 0.2-36) in the 31 patients age >70 years (P = .027).
Responses at week 23 in the ITT population according to either local investigators or IRR
| . | Local investigators, n (%) . | IRR, n (%)* . | ||
|---|---|---|---|---|
| R (n = 77) . | RL (n = 77) . | R (n = 77) . | RL (n = 77) . | |
| CR/CRu | 19 (25) | 28 (36) | 28 (36) | 47 (61) |
| 95% CI, % | 16-36 | 26-48 | 26-48 | 49-72 |
| PR | 28 (36) | 35 (45) | 16 (21) | 13 (17) |
| SD | 6 (8) | 4 (5) | 7 (9) | 2 (3) |
| PD | 2 (3) | 3 (4) | 3 (4) | 1 (1) |
| NE† | 22 (29) | 7 (9) | 23 (30) | 14 (18) |
| . | Local investigators, n (%) . | IRR, n (%)* . | ||
|---|---|---|---|---|
| R (n = 77) . | RL (n = 77) . | R (n = 77) . | RL (n = 77) . | |
| CR/CRu | 19 (25) | 28 (36) | 28 (36) | 47 (61) |
| 95% CI, % | 16-36 | 26-48 | 26-48 | 49-72 |
| PR | 28 (36) | 35 (45) | 16 (21) | 13 (17) |
| SD | 6 (8) | 4 (5) | 7 (9) | 2 (3) |
| PD | 2 (3) | 3 (4) | 3 (4) | 1 (1) |
| NE† | 22 (29) | 7 (9) | 23 (30) | 14 (18) |
NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
On imaging only.
Patients NE at week 23 included those already showing PD or not achieving at least an MR at week 10 and those who discontinued before week 23 because of unacceptable toxicity or any other reason, as indicated in the CONSORT diagram (Figure 1). These patients were counted as nonresponders. In 8 patients, CT scan imaging was not available for IRR.
According to the multivariable analysis performed to explore the effects of the stratification factors, response did not depend on FLIPI score or tumor bulk or grade; however, PFS was longer in patients with favorable FLIPI scores (Table 3).
Logistic multiple regression analysis of CR/CRu at week 23 and Cox proportional hazard model for PFS
| Effect . | Logistic regression analysis of CR/CRu at week 23 . | Cox proportional hazards model for PFS . | ||||
|---|---|---|---|---|---|---|
| P . | OR . | 95% CI . | P . | HR . | 95% CI . | |
| Treatment, RL vs R | .1094* | 1.772 | 0.880-3.570 | .0413 | 0.611 | 0.380-0.981 |
| Grade, 3A vs 1 or 2 | .5797 | 1.296 | 0.518-3.244 | .9351 | 0.973 | 0.506-1.872 |
| Bulky disease, yes vs no | .2672 | 0.663 | 0.321-1.370 | .9262 | 0.977 | 0.601-1.588 |
| Risk by FLIPI score, high vs low or intermediate | .9979 | 0.999 | 0.497-2.008 | .0113 | 1.845 | 1.148-2.962 |
| Effect . | Logistic regression analysis of CR/CRu at week 23 . | Cox proportional hazards model for PFS . | ||||
|---|---|---|---|---|---|---|
| P . | OR . | 95% CI . | P . | HR . | 95% CI . | |
| Treatment, RL vs R | .1094* | 1.772 | 0.880-3.570 | .0413 | 0.611 | 0.380-0.981 |
| Grade, 3A vs 1 or 2 | .5797 | 1.296 | 0.518-3.244 | .9351 | 0.973 | 0.506-1.872 |
| Bulky disease, yes vs no | .2672 | 0.663 | 0.321-1.370 | .9262 | 0.977 | 0.601-1.588 |
| Risk by FLIPI score, high vs low or intermediate | .9979 | 0.999 | 0.497-2.008 | .0113 | 1.845 | 1.148-2.962 |
P value corresponding to a 2-sided test has to be divided by 2 for a 1-sided test (.0547) and is statistically significant according to the predefined 1-sided type 1 error of 0.10 for the primary end point of CR/CRu at week 23.
Of the 23 patients who attained an MR at week 10 in the R arm, only 12 achieved a better response at week 23 (Cru, n = 1; partial response, n = 11), whereas in the RL arm, response at week 23 improved in 10 of 14 MR patients (Cru, n = 2; partial response, n = 8).
Sensitivity analysis, using response assessment from IRR, comparing baseline and week 23 CT scans also showed a significantly higher CR/CRu rate in the RL arm, both in the ITT population (61% [95% CI: 49%-72%] vs 36% [95% CI: 26%-48%], P = .001) and the per-protocol population (67% [95% CI: 53%-79%] vs 40% [95% CI: 29%-53%], P = .001). Waterfall plots comparing the CT scan evaluations performed independently by the 2 external experts and by the trial investigators showed similar response patterns (supplemental Figure 1).
The CR/CRu improvement achieved with the addition of L was maintained over time. Follow-up exceeded 30 months for all patients, and the CR30, recently identified as a reliable surrogate of PFS,26 was significantly better in the combination arm (42% [95% CI: 30%-53%] vs 19% [95% CI: 11%-30%], P = .001).
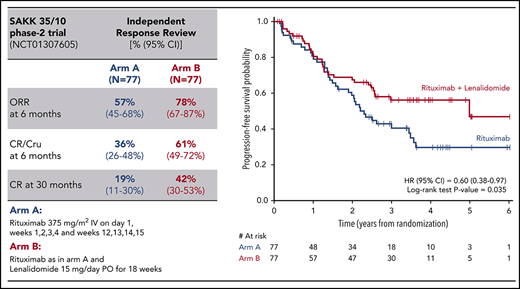
Figure 2 shows the Kaplan-Meier estimates of time-to-event end points; at a median follow-up of 4 years, a longer median PFS (5.0 vs 2.3 years; HR, 0.60 [95% CI: 0.38-0.97], P = .035) and a longer DOR were observed for the patients in the combination arm (median not reached vs 3.0 years; HR, 0.46 [95% CI: 0.20-1.04], P = .055). TTNT was also significantly longer with the combination (median not reached vs 2.1 years; HR, 0.49 [95% CI: 0.30-0.78], P = .003).
Kaplan-Meier curves of secondary (time-dependent) end points with correlated HRs.
Kaplan-Meier curves of secondary (time-dependent) end points with correlated HRs.
Individual tumor sensitivity to R or RL was assessed at week 10, allowing patients with insufficient response or failure resulting from toxicity to receive alternative therapy, at the discretion of the local investigator. Patients with a later progression or relapse received therapy when indicated. At the time of the analysis, 46 patients (60%) treated in the R arm had started a new antilymphoma therapy, compared with 30 patients (39%) in the RL arm. A few patients received R as maintenance (n = 4) or radiotherapy as consolidation (n = 1), which was regarded as protocol violation and not counted as an event for TTNT. These patients (R arm, n = 2; RL arm, n = 3) were censored at the time point of radiotherapy/start of R maintenance. In 11 patients, local radiotherapy (n = 8) or R (n = 3) administered after progression was included as an event in TTNT.
Details of second-line treatments after trial therapy and their subsequent outcomes are listed in Table 4; a trend toward a higher CR/CRu rate was apparent for the R arm (P = .067), but when limiting the analysis to the subset of patients treated at relapse with either R-CHOP (R plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-bendamustine, overall remission rates and CR/CRu rates were not significantly different (data not shown). Additional treatments are described in the supplemental data.
Second-line treatments
| . | R (n = 77), n (%) . | RL (n = 77), n (%) . | Total (N = 154), n (%) . |
|---|---|---|---|
| First new therapy after trial treatment | 46 (60) | 30 (39) | 76 (49) |
| Treatment type | |||
| R-bendamustine* | 14 (30) | 11 (37) | 24 (32) |
| R-CHOP | 10 (22) | 7 (23) | 17 (22) |
| R-chlorambucil† | 4 (9) | 4 (13) | 8 (11) |
| R-CVP | 3 (7) | 1 (3) | 4 (5) |
| R alone‡ | 2 (4) | 5 (17) | 7 (9) |
| Radiotherapy alone§ | 7 (15) | 2 (7) | 9 (12) |
| High-dose therapy/autologous SCT | 2 (4) | 0 | 2 (3) |
| Other‖ | 4 (9) | 0 | 4 (5) |
| Response after first new therapy | |||
| CR/CRu | 18 (39) | 7 (23) | 25 (33) |
| PR | 20 (43) | 11 (37) | 31 (41) |
| SD | 1 (2) | 2 (7) | 3 (4) |
| PD | 5 (11) | 5 (17) | 10 (13) |
| Not assessable | 2 (4) | 5 (17) | 7 (9) |
| . | R (n = 77), n (%) . | RL (n = 77), n (%) . | Total (N = 154), n (%) . |
|---|---|---|---|
| First new therapy after trial treatment | 46 (60) | 30 (39) | 76 (49) |
| Treatment type | |||
| R-bendamustine* | 14 (30) | 11 (37) | 24 (32) |
| R-CHOP | 10 (22) | 7 (23) | 17 (22) |
| R-chlorambucil† | 4 (9) | 4 (13) | 8 (11) |
| R-CVP | 3 (7) | 1 (3) | 4 (5) |
| R alone‡ | 2 (4) | 5 (17) | 7 (9) |
| Radiotherapy alone§ | 7 (15) | 2 (7) | 9 (12) |
| High-dose therapy/autologous SCT | 2 (4) | 0 | 2 (3) |
| Other‖ | 4 (9) | 0 | 4 (5) |
| Response after first new therapy | |||
| CR/CRu | 18 (39) | 7 (23) | 25 (33) |
| PR | 20 (43) | 11 (37) | 31 (41) |
| SD | 1 (2) | 2 (7) | 3 (4) |
| PD | 5 (11) | 5 (17) | 10 (13) |
| Not assessable | 2 (4) | 5 (17) | 7 (9) |
PD, progressive disease; PR, partial response; R-CHOP, R plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, R plus cyclophosphamide, vincristine, and prednisone; SCT, stem cell transplantation; SD, stable disease.
Including 1 patient receiving obinutuzumab and bendamustine (RL arm) and 3 receiving bendamustine alone (R arm, n = 2; RL arm, n = 1).
Including 1 patient receiving chlorambucil and prednisone (RL arm).
Administered either as new therapy after relapse/progression (R arm, n = 1; RL arm, n = 2) or as consolidation/maintenance after the trial treatment (R arm, n = 1; RL arm, n = 3).
Administered as new therapy after relapse/progression in all cases, with the exception of 1 patient in R arm who received consolidation radiotherapy after the trial treatment.
Including 1 patient receiving BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) for Hodgkin lymphoma and 3 patients receiving experimental treatments in other clinical trials.
A total of 14 deaths were recorded: 7 in the R arm (lymphoma, n = 4; pulmonary embolism, n = 1; and cardiac events, n = 2) and 7 in the RL arm (lymphoma, n = 6; accident, n = 1). OS was not statistically different in the 2 arms; the 4-year OS rate was 91% (95% CI, 81%-96%) in the combination arm and 90% (95% CI, 79%-95%) in the R arm (Figure 2).
Safety
Adverse effects in both arms are summarized in Table 5. Adverse events of any grade were reported in 100% of patients in the combination arm and 91% in the R arm. Grade ≥3 adverse events were also more common with the combination regimen (56% vs 22% of patients). Grade 3 to 4 neutropenia was observed in 23% of patients receiving RL and 7% of those treated with R; this was febrile neutropenia in 1 patient in the R arm and no patients in the combination arm. There were no trial treatment-related deaths in any arm. Fatigue, diarrhea, and skin rash were more common in the combination arm; however, these adverse effects were mostly grade 1 to 2 (grade 3 was observed in ≤5% in both arms, and no grade 4 was reported). Eleven patients stopped L for unacceptable toxicity: rash (grade 2, n = 2; grade 3, n = 3), thrombosis (grade 2, n = 1), atrial fibrillation (grade 2, n = 1), Steven-Johnson syndrome (grade 3, n = 1), hyponatremia (grade 3, n = 1), hematological toxicity (grade 3, n = 1), and abdominal pain (grade 3, n = 1). Only 1 patient stopped treatment for unacceptable toxicity (oral mucositis, generalized edema, and hives; all grade 3) in the R monotherapy arm.
Adverse events
| Adverse Event . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R (n = 76), n (%) . | RL (n = 77), n (%) . | |||||||||
| Any . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Any . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| Hematological | ||||||||||
| Neutropenia | 4 (5) | 3 (4) | 1 (1) | 18 (23) | 14 (18) | 4 (5) | ||||
| Lymphopenia | 1 (1) | 1 (1) | ||||||||
| Anemia | 2 (3) | 2 (3) | 3 (4) | 1 (1) | 1 (1) | 1 (1) | ||||
| Thrombocytopenia | 4 (5) | 1 (1) | 2 (3) | 1 (1) | ||||||
| Febrile neutropenia | 1 (1) | 1 (1) | ||||||||
| Nonhematological | ||||||||||
| IR symptoms | 11 (14) | 2 (3) | 8 (11) | 1 (1) | 5 (6) | 1 (1) | 4 (5) | |||
| Fatigue | 26 (34) | 17 (22) | 8 (11) | 1 (1) | 40 (52) | 29 (38) | 9 (12) | 2 (3) | ||
| Fever | 11 (14) | 7 (9) | 4 (5) | 12 (16) | 11 (14) | 1 (1) | ||||
| Diarrhea | 9 (12) | 6 (8) | 3 (4) | 19 (25) | 18 (23) | 1 (1) | ||||
| Nausea/vomiting | 12 (16) | 8 (11) | 4 (5) | 17 (22) | 14 (18) | 2 (3) | 1 (1) | |||
| Mucositis | 5 (7) | 4 (5) | 1 (1) | 3 (4) | 2 (3) | 1 (1) | ||||
| Skin rash | 5 (7) | 2 (3) | 2 (3) | 1 (1) | 21 (27) | 11 (14) | 6 (8) | 4 (5) | ||
| Cough | 10 (13) | 9 (12) | 1 (1) | 19 (25) | 14 (18) | 5 (6) | ||||
| Transaminase increase | 2 (3) | 1 (1) | 1 (1) | 2 (3) | 1 (1) | 1 (1) | ||||
| Headache | 7 (9) | 5 (7) | 2 (3) | 12 (16) | 12 (16) | |||||
| Infections | 14 (18) | 6 (8) | 6 (8) | 2 (3) | 23 (30) | 6 (8) | 14 (18) | 3 (4) | ||
| Skin infection | 2 (3) | 1 (1) | 1 (1) | 4 (5) | 2 (3) | 2 (3) | ||||
| Upper respiratory infection | 8 (11) | 6 (8) | 2 (3) | 13 (17) | 4 (5) | 8 (10) | 1 (1) | |||
| Urinary tract infection | 3 (4) | 3 (4) | 4 (5) | 2 (3) | 2 (3) | |||||
| Other* | 1 (1) | 1 (1) | 4 (5) | 1 (1) | 3 (4) | |||||
| Adverse Event . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R (n = 76), n (%) . | RL (n = 77), n (%) . | |||||||||
| Any . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Any . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| Hematological | ||||||||||
| Neutropenia | 4 (5) | 3 (4) | 1 (1) | 18 (23) | 14 (18) | 4 (5) | ||||
| Lymphopenia | 1 (1) | 1 (1) | ||||||||
| Anemia | 2 (3) | 2 (3) | 3 (4) | 1 (1) | 1 (1) | 1 (1) | ||||
| Thrombocytopenia | 4 (5) | 1 (1) | 2 (3) | 1 (1) | ||||||
| Febrile neutropenia | 1 (1) | 1 (1) | ||||||||
| Nonhematological | ||||||||||
| IR symptoms | 11 (14) | 2 (3) | 8 (11) | 1 (1) | 5 (6) | 1 (1) | 4 (5) | |||
| Fatigue | 26 (34) | 17 (22) | 8 (11) | 1 (1) | 40 (52) | 29 (38) | 9 (12) | 2 (3) | ||
| Fever | 11 (14) | 7 (9) | 4 (5) | 12 (16) | 11 (14) | 1 (1) | ||||
| Diarrhea | 9 (12) | 6 (8) | 3 (4) | 19 (25) | 18 (23) | 1 (1) | ||||
| Nausea/vomiting | 12 (16) | 8 (11) | 4 (5) | 17 (22) | 14 (18) | 2 (3) | 1 (1) | |||
| Mucositis | 5 (7) | 4 (5) | 1 (1) | 3 (4) | 2 (3) | 1 (1) | ||||
| Skin rash | 5 (7) | 2 (3) | 2 (3) | 1 (1) | 21 (27) | 11 (14) | 6 (8) | 4 (5) | ||
| Cough | 10 (13) | 9 (12) | 1 (1) | 19 (25) | 14 (18) | 5 (6) | ||||
| Transaminase increase | 2 (3) | 1 (1) | 1 (1) | 2 (3) | 1 (1) | 1 (1) | ||||
| Headache | 7 (9) | 5 (7) | 2 (3) | 12 (16) | 12 (16) | |||||
| Infections | 14 (18) | 6 (8) | 6 (8) | 2 (3) | 23 (30) | 6 (8) | 14 (18) | 3 (4) | ||
| Skin infection | 2 (3) | 1 (1) | 1 (1) | 4 (5) | 2 (3) | 2 (3) | ||||
| Upper respiratory infection | 8 (11) | 6 (8) | 2 (3) | 13 (17) | 4 (5) | 8 (10) | 1 (1) | |||
| Urinary tract infection | 3 (4) | 3 (4) | 4 (5) | 2 (3) | 2 (3) | |||||
| Other* | 1 (1) | 1 (1) | 4 (5) | 1 (1) | 3 (4) | |||||
Adverse events were reported from study entry to last tumor assessment at week 23 or 30 d after treatment discontinuation or immediately before any off-trial treatment, whichever occurred first. Serious adverse events possibly related to late toxicity of the study treatment and second malignancies occurring during the follow-up had to be reported according to the protocol.
Including 1 venous catheter infection in R arm and 1 grade 1 viral infection, 2 grade 2 dental abscesses, and 1 grade 2 infection of unclear origin in RL arm.
Transformation and secondary malignancies
Two biopsy-proven transformations into diffuse large B-cell lymphoma were reported, both in the combination arm. Two Hodgkin lymphomas (clonal relation with FL was not investigated) and 1 prostate cancer were documented in the R arm, and 1 prostate cancer, 1 adenocarcinoma in situ of the lung, and 3 skin cancers (squamous cell, n = 2; basal cell, n = 1) occurred in the RL arm.
Discussion
Long-term results of randomized studies have shown that front-line R monotherapy is efficacious and safe in advanced and symptomatic FL, suggesting that chemotherapy can be deferred in a significant proportion of patients without compromising outcomes21,23,37 and that R alone may still be considered as a benchmark for the evaluation of novel chemotherapy-free regimens.25 The SAKK 35/10 trial is the first randomized trial in previously untreated patients with FL demonstrating that the addition of L to R is associated with significantly better response rates and PFS than R monotherapy. Importantly, the improved results achieved with RL were not associated with any unexpected toxicity. The adverse effects were manageable and seemingly fewer in number and of less severity compared with those reported with immunochemotherapy. Our study is unique in comparison with the other published phase 2 trials of RL because of its randomized design, patient population (all untreated and requiring treatment), and short duration of therapy (<6 vs >12 months in other studies).29-31,38
Inclusion of symptomatic patients in need of therapy only in our trial resulted in a high proportion of patients with advanced-stage disease and poor-risk FLIPI scores, a population similar to those of large registration front-line immunochemotherapy phase 3 trials.16,39 Compared with the pivotal MD Anderson single-arm trial testing the RL combination in untreated FL,29 our patients had higher median age (62 vs 56 years), and more patients in our study (47% vs 28%) had poor-risk FLIPI scores. Moreover, in this previous trial, the criteria for treatment initiation were not specified, and the treatment schedule was different from that used in our trial.
The design of our randomized phase 2 trial (short treatment, no maintenance) aimed to reach a faster proof of principle before designing subsequent phase 3 studies. Accordingly, adoption of a 1-sided test with a false-positive rate of 10% was considered an appropriate option to explore treatment benefit while containing the sample size.35,36 The chosen R schedule had already been shown to be efficacious in the prior studies of the Nordic group, which reported that 42% of responders to the first 4 doses of R were still failure free at 5 years,20,22 with an OS rate of 78% at 10 years from diagnosis23 and OS comparable to that achieved with immunochemotherapy.40
In our RL trial, L was administered for 2 weeks before adding R, following the hypothesis generated by preclinical data that priming with L may enhance R activity against CD20+ tumor cells in vivo.27,28 The lenalidomide schedule was chosen to have a continuous immunomodulatory drug activity while maintaining the same monthly dose used in prior studies.29,38
The primary end point was met,35 with a significantly better CR/CRu rate in the combination arm compared with R monotherapy. This benefit seemed higher in the IRR than in the investigator assessment, mostly depending on the fact that the IRR evaluated only the target lesions measurable on CT scans, whereas the investigator assessment required the ascertained normalization of all lymphoma-related parameters (bone marrow, lactate dehydrogenase, and other laboratory or clinical abnormalities) to define response. Waterfall plots of CT scan evaluations by investigators and the IRR were indeed similar.
Our chemotherapy-free approach was also effective in patients with advanced-stage bulky disease and poor-risk FLIPI score and in patients age >70 years. Survival was excellent, with no differences between the treatment arms. As expected, toxicity, both hematological and nonhematological, was higher in the combination arm; however, there were few grade 3 or 4 nonhematological events. The rates of the most common grade 3 or 4 events observed with the combination regimen (neutropenia in 23% and skin rash in 5% of the patients) in our study were in the same range as rates in other studies of patients not previously treated.29,38,41 The rate of treatment discontinuation for nonsevere toxicities seemed to decrease throughout the study, indicating a possible learning curve in the management of adverse effects.
In comparison with treatments in other FL trials,29,38 our treatment regimen was shorter at 15 weeks in total (18 weeks in the combination arm). However, at the end of induction, the observed CR rate was in the same range as the rate in the GALLIUM trial,39 a first-line R-chemotherapy trial, but clearly inferior to the responses reported by Fowler and Martin29,38 using an RL combination. This suggests that patient characteristics, particularly the presence of symptomatic disease requiring therapy, affect outcomes, and comparisons between different trials should be evaluated with caution. In our trial, all patients were symptomatic, and response (CR, CRu, or MR at week 10) was required to continue the study treatment, because most nonresponding patients were in need of alternative treatment. This may have biased the results, because responses might have been slower in the single-agent arm. However, our findings with RL are in line with those reported in previously published phase 2 trials.29,38 Moreover, the use of positron emission tomography in some of these trials of RL may also explain their higher CR rates; unfortunately, when our study was designed, positron emission tomography/CT was not widely accepted as a restaging tool in FL and could not be used. Nevertheless, we cannot exclude that the shorter duration of treatment in our trial may have contributed to the lower CR rate. It should be noted, however, that the shorter schedule did not show any detrimental effect on OS. Our early evaluation of response may also have affected the CR rate, because responses continued to improve over time.
The study was not powered to allow subgroup analysis; nevertheless, we assessed the impact of treatment in older patients, because they may represent a suitable population for nonchemotherapy treatments. In our study, the activity of the RL combination remained significantly higher in patients age >70 years, a group of patients in whom administration of standard immunochemotherapy is often difficult. Moreover, older patients may particularly benefit from shorter treatment. Quality of life and patient-reported outcomes could not be assessed in our trial, which may be regarded as a potential limitation.
The median PFS in our trial was twice as long in patients in the RL arm compared with the R monotherapy arm; TTNT was also longer in the combination arm. However, the lack of maintenance prevents any comparison with a majority of other studies.
Second-line immunochemotherapy was required in 60% of the patients in the R arm and 39% of those in the RL arm. Approximately three-quarters of the patients responded to second-line treatments, with an expected trend for higher remission rates in patients initially treated with R monotherapy; nonetheless, OS rates seem thus far to be similar in both arms.
Final results were recently made available from the AUGMENT (ClinicalTrials.gov identifier: NCT01938001) and RELEVANCE (ClinicalTrials.gov Identifier: NCT01650701) trials, which compared RL with R alone in pretreated patients and RL with immunochemotherapy in treatment-naïve patients, respectively.41,42 Analogous to what we found in the front-line setting, the AUGMENT study showed superior efficacy of the combination over R monotherapy in relapsing/refractory patients. In the RELEVANCE study, the front-line administration of the RL combination with maintenance (L for 1 and R for 2 years) led to a CR/CRu rate of 48% at 30 months (95% CI, 44%-53%), slightly higher than the 42% rate (95% CI, 30%-53%) obtained with our shorter schedule. Also, 3-year PFS was better in the RELEVANCE study (77%; 95% CI, 72%-80%) compared with ours (56%; 95% CI, 43%-67%), which is not surprising, because R maintenance is known to be significantly beneficial in FL. In the RELEVANCE trial, the RL regimen was not superior to R-chemotherapy (plus R maintenance), and PFS was superimposable in both arms. Both trials are therefore suggesting that the RL combination is a promising option for FL front-line therapy.41 The RELEVANCE study also suggests that a more prolonged schedule may result in better outcomes. Our trial seems to indicate that a shorter and possibly better tolerated regimen induced durable responses in three-quarters of the treated patients, but the study design does not allow conclusions to be drawn about the optimal duration of RL treatments.
In conclusion, the results of our study support the growing evidence that RL regimens might offer an alternative to standard immunochemotherapy. The acceptable and manageable toxicity and the excellent OS in both arms suggest that chemotherapy-free strategies should further be explored, even if the costs of novel targeted agents remain an important unsolved issue, particularly when OS is apparently unaffected by the treatment.
Presented in part at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA, 9 December 2014; the 58th Annual Meeting of the American Society of Hematology, San Diego, CA, 5 December 2016; and the 13th International Conference on Malignant Lymphoma, Lugano, Switzerland, 17 June 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank patients, coinvestigators, and research nurses and data managers at each of the participating centers for their invaluable contribution, as well as the central study team at the SAKK coordinating center for their administrative support and assistance in data collection and study conduction. Furthermore, the authors thank Verena Voelter at Celgene International for her important help in the initial study development.
The trial was supported by the Swiss State Secretariat for Education, Research and Innovation and Celgene International Sàrl.
Authorship
Contribution: E.K., S.B.V., and E.Z. designed the study, performed research, and analyzed the data; S.R. performed statistical analysis and contributed to manuscript writing; E.K. and E.Z. wrote the paper; S.B.V. coordinated the administrative conduct of the trial and critically reviewed the manuscript; S.H. contributed to statistical analysis; and all authors contributed to patient management and data collection, reviewed and approved the manuscript, and shared final responsibility for the decision to submit.
Conflict-of-interest disclosure: E.Z. has received research funding from Celgene, Roche, and Janssen, served on the advisory boards of Celgene, Roche, Mei Pharma, AstraZeneca, and Celltrion Healthcare, received travel grants to meetings from AbbVie and Gilead, and provided expert statements to Gilead, Bristol-Myers Squibb, and MSD. B.Ø. has served on the advisory board of Celgene. U.J.M.M. has served on the advisory boards of and received research funding from Roche and Celgene. M.H. has served on the advisory boards of MSD, Bristol-Myers Squibb, Amgen, Incyte, Sanofi, and Novartis, given lectures to MSD, Novartis, and Bristol-Myers Squibb, and received travel grants to meetings from Amgen, MSD, Sanofi, Roche, and Novartis. A.J.M.F has received research funding from Celgene. M.G. has received honoraria from Roche, Mundipharma, Celgene, AbbVie, Gilead, and Sandoz. E.K. has served on the advisory boards of Roche, Celgene, Janssen, AbbVie, Pfizer, Mei Pharma, and Sandoz, provided educational lectures for Gilead, Roche, Celgene, Janssen, AbbVie, and Roche, received research funding from Pfizer. The remaining authors declare no competing financial interests.
Complete lists of the members of the Swiss Group for Clinical Cancer Research and the Nordic Lymphoma Group appear in "Appendix."
Correspondence: Emanuele Zucca, IOSI Ospedale San Giovanni, CH-6500 Bellinzona, Switzerland; e-mail: emanuele.zucca@eoc.ch.
Appendix: study group members
The members of the Swiss Group for Clinical Cancer Research are M.B., Clemens Caspar, Dieter Köberle, F.K., E.Z., Urban Novak, Reinhard Zenhäusern, Lorenz M. Jost, U.J.M.M., Nicolas Mach, Michèle Voegeli, T.Z., W.M., F.H., D.R., Georg Tscherry, Natalie Fischer, Roger Burkhard, Mathias Schmid, and Samaras Panagiotis. The members of the Nordic Lymphoma Group are P.d.N.B., Lars Munksgaard, Micaela Hernberg, Kaija Vasala, Tuula Lehtinen, Sirkku Jyrkkiö, Roald Ekanger, Jürgen Rolke, B.Ø., Peter Meyer, Martin Maisenhölder, Monika Eidem, Anders Radlund, Ingemar Lagerlöf, Lena Brandefors, Ola Lindén, Kristina Arnljots, Eva Kimby, Maria Strandberg, A.-S.J., and H.H.