TO THE EDITOR:
Diagnosis of amyloidosis, with the possible exception of cardiac transthyretin amyloidosis (ATTR) in patients without monoclonal components,1 requires demonstration of tissue amyloid deposits and identification of the amyloidogenic protein.2,3 The gold standard for amyloid detection is Congo red (CR) staining, based on amyloid’s congophilia and birefringence under polarized light (PL; CR-PL).4 When systemic amyloidosis is suspected, subcutaneous abdominal fat, acquired by fine-needle aspiration or punch biopsy, is considered the tissue of choice, given the high sensitivity (up to 80% to 90%)5-7 and low risk of major complications.5
Although birefringence under PL is a defining feature of amyloid,2 CR-PL interpretation critically depends on equipment quality, the pathologist’s expertise, and connective tissue abundance.8,9 This may translate into low specificity in nonspecialized centers and unnecessary second-level testing. In our experience, 85% of the >300 putatively CR+ fat specimens sent to our Center for amyloid typing were reclassified as negative after performing and evaluating CR staining in-house and with immuno–electron microscopy (IEM). Novel amyloid-binding compounds were developed as possible complements to CR in histology.10-14 A major challenge is to achieve the clinical performances of CR, while allowing for easier staining and results interpretation. Examples of new dyes include luminescent-conjugated oligothiophenes,10-12 and the fluorescent CR analogs (E,E),-1-bromo-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (BSB)13,14 and (E,E)-1-fluoro-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (FSB).14,15 In particular, FSB was shown to bind amyloid specifically in tissues and in vivo16 and to stain various amyloid types, with stronger fluorescence than CR and BSB. In pilot studies, FSB showed higher sensitivity than CR.14,15,17 These dyes, however, have not yet reached the clinical routine, and systematic studies on large patient cohorts are lacking. Herein, we prospectively assessed the usefulness of FSB to detect amyloid in fat aspirates from individuals with suspected systemic amyloidosis, through comparison of FSB results against CR-PL.
Consecutive individuals referred to the Pavia Amyloidosis Center (December 2017 to July 2018) were enrolled. Written informed consent was obtained for use of biological samples and clinical data for research, according to the institutional review board guidelines. Patients underwent clinical, instrumental, and genetic examination as described.6 In cases without evidence of amyloid deposits in fat but persistent clinical suspicion of amyloidosis, biopsy of affected organs was performed to confirm or exclude diagnosis. Subcutaneous periumbilical fat was acquired by fine-needle aspiration; samples (∼50 mg) were split into 3 comparable parts to be examined by IEM,6 FSB, and CR-PL (the latter was stained and evaluated as described4,6 by 2 expert physicians blinded to FSB results). For FSB staining, samples were immediately washed 3 times with cold phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde (1 hour), and immersed in FSB working solution (0.01% vol/vol in PBS; 2 hours) (Sigma-Aldrich/Merck). Specimens were washed 4 times with PBS (5 minutes each; agitation), placed on histology slides, overlaid with ProLong Gold (Thermo Fisher Scientific), and squeezed under a cover slide. Fluorescence was visualized using a Olympus Fluoview FV10i-LIV confocal microscope (excitation, 405 nm; emission, 420-520 nm). Negative and positive controls were used to set microscope parameters that allowed visualization of the FSB signal without background autofluorescence.
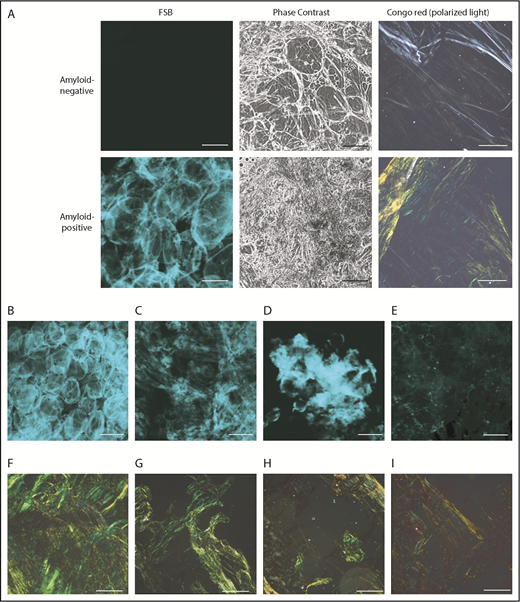
Fat smears were evaluated by CR-PL and FSB in 206 individuals. FSB positivity is defined by presence of a fluorescent extracellular signal above background.16 (Figure 1); the dye’s strong fluorescence translates into high contrast between amyloid areas and background, so that even tiny amounts of FSB+ material are spotted. Signal from collagen, other tissue structures, and blood is absent. Amyloid amount and FSB signal intensity differed across patients, but recurrent patterns were observed (Figure 1B-E): pericellular deposition (Figure 1B), diffuse interstitial (Figure 1C) or focal (Figure 1D) accumulation, and multiple tiny foci (Figure 1E). Avoiding deposition of dust or fabric debris is important because they become visible as single fluorescent dots/stripes with sharp contours; their signal, however, is typically not of the same focus as cells.
Representative amyloid-positive and -negative subcutaneous abdominal samples, stained with the CR–derivative FSB. (A) Paired images from amyloid-positive and amyloid-negative samples, acquired under confocal microscopy and phase-contrast analysis. (B-E) Distinct patterns of amyloid deposition (corresponding CR-PL images are visible, respectively, in panels F-I). (B) Pericellular distribution (ALλ patient). (C) Diffuse interstitial distribution (ALλ patient). (D) Focal interstitial (ATTRwt patient). (E) Tiny scattered amyloid foci (ALλ patient). Scale bars, 100 μm in all FSB panels; 200 μm in all CR panels. In the pericellular type, amyloid is distributed around cells and defines the contour of the adipocytes, whereas the interstitium is not enlarged. In contrast, in diffuse and focal interstitial types, large (widespread or localized, respectively) accumulations of amyloid are seen between cells. In the last type (E), tiny amyloid deposits are scattered across the sample in the interstitial space and within vessels.
Representative amyloid-positive and -negative subcutaneous abdominal samples, stained with the CR–derivative FSB. (A) Paired images from amyloid-positive and amyloid-negative samples, acquired under confocal microscopy and phase-contrast analysis. (B-E) Distinct patterns of amyloid deposition (corresponding CR-PL images are visible, respectively, in panels F-I). (B) Pericellular distribution (ALλ patient). (C) Diffuse interstitial distribution (ALλ patient). (D) Focal interstitial (ATTRwt patient). (E) Tiny scattered amyloid foci (ALλ patient). Scale bars, 100 μm in all FSB panels; 200 μm in all CR panels. In the pericellular type, amyloid is distributed around cells and defines the contour of the adipocytes, whereas the interstitium is not enlarged. In contrast, in diffuse and focal interstitial types, large (widespread or localized, respectively) accumulations of amyloid are seen between cells. In the last type (E), tiny amyloid deposits are scattered across the sample in the interstitial space and within vessels.
Overall, CR-PL and FSB provided concordant results in 204 of 206 cases (99%; 95% confidence interval [CI], 96.5% to 99.7%). Of these, 135 were classified as negative and 69 as positive by both. Amyloid was typed by IEM in all CR+ cases; diagnoses included: 1 reactive (AA), 3 wild-type (ATTRwt), and 2 variant (ATTRv) ATTR, and 63 light-chain (AL) amyloidoses (9 ALκ, 54 ALλ). Through organ biopsy, systemic amyloidosis was confirmed in 43 of 135 CR−/FSB− cases (9 AL, 1 AA, 5 ATTRv, 28 ATTRwt) and was excluded in 66. Twenty-six patients had localized amyloidosis (skin, upper respiratory tract, lung nodular, gastrointestinal tract).
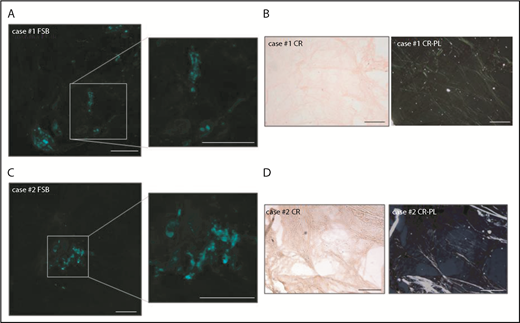
The 2 samples in which FSB and CR-PL disagreed were FSB+/CR− (Figure 2). In case 1, the FSB signal consisted of scattered dots (Figure 2A). In case 2, a single positive area was detected. In both, fat-pad IEM analysis was positive and allowed for the typing of fibrils, respectively, as ALλ and ATTR. In case 2, ATTRwt was then diagnosed based on positive 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) scintigraphy in the absence of monoclonal components and TTR gene mutations. Finally, in FSB+/CR− samples, we explored whether CR fluorescence could increase sensitivity,18,19 but the high background prevented classification. Considering all patients who finally received a diagnosis of systemic amyloidosis (of whom 69 were CR+/FSB+, 43 CR−/FSB−, 2 CR−/FSB+), overall sensitivity of CR-PL was 60.5% (95% CI, 51.3% to 69%) (86.3% for AL; 9.4% for ATTRwt; 28.6% for ATTRv) and sensitivity of FSB was 62.3% (95% CI, 53% to 70.6%) (87.7% for AL; 12.5% for ATTRwt; 28.6% for ATTRv).
Details of the 2 FSB+/CR−adipose tissue samples. (A,C) FSB-stained tissue from cases 1 and 2, respectively (low magnification images and enlarged insets). (B,D) CR-stained tissue from cases 1 and 2, respectively (left, nonpolarized light; right, polarized light). Scale bars, 200 μm.
Details of the 2 FSB+/CR−adipose tissue samples. (A,C) FSB-stained tissue from cases 1 and 2, respectively (low magnification images and enlarged insets). (B,D) CR-stained tissue from cases 1 and 2, respectively (left, nonpolarized light; right, polarized light). Scale bars, 200 μm.
This is the first large prospective study evaluating the diagnostic sensitivity of the CR derivative FSB for amyloid detection in fat smears. Comparable diagnostic sensitivity and almost 100% concordance between FSB and CR was demonstrated in an unselected population of consecutive individuals with suspected systemic amyloidosis. CR-PL sensitivity was in agreement with previous reports.6,7 Discordant cases were 2, <1%, and, in both, IEM confirmed FSB results. FSB is commercially available with contained cost and presents convenient features that make it an attractive candidate for clinical implementation. The staining protocol is fast (3.5 hours), requires little sample handling and avoids manipulation of CR powder, which is a suspected carcinogen. Procedure implementation is straightforward and results evaluation is less dependent on subjective interpretation compared with CR-PL. FSB+ areas stand out from background, even when deposits consist of scattered dots (Figure 1E). Examining adequately large specimens (at least ∼3-4 mm side) is critical to increasing the chance of detecting sparse deposits. Signal from other extracellular structures is absent, overcoming the confounding effect of collagen and blood, which can affect CR-PL interpretation. In our experience, fluorescence microscopy provides results consistent with confocal microscopy17 ; future dedicated studies will help define the interchangeability of the 2 techniques. Overall, FSB is a promising method for allowing rapid, sensitive, reliable amyloid detection on fat aspirates, provided that fluorescence or confocal microscopy is available; this could translate into faster diagnosis and more appropriate referral to specialized centers for typing and treatment.
The optimal signal-to-background ratio also makes FSB attractive for applications where fluorescence of amyloid-binding dyes is commonly exploited, including laser microdissection coupled to mass spectrometry,20,21 in which it could improve selection of regions to be dissected. We also anticipate that the specific fluorescence and low background of FSB could be exploited for amyloid typing: in fact, FSB colocalization with fluorescently labeled antibodies targeting amyloid proteins could be objectively quantified, reducing the confounding impact of tissue autofluorescence, serum contamination, and nonspecific antibody binding.
FSB staining is a promising approach for amyloid detection in fat aspirates. Validating its clinical performance and procedure harmonization through multicenter studies will allow the establishment of its role as a possible complement or alternative to CR.
Acknowledgments
The authors thank Roberta Riboni for assistance with fluorescence microscopy.
This work was supported by Fondazione Cariplo (grant nos. 2014-0700, 2015-0591, 2016-0489), Associazione Italiana per la Ricerca sul Cancro special program “5 per mille” (no. 9965), the Italian Ministry of Health (grants RF-2013-02355259 and RF-2016-02361756), the Italian Medicines Agency (grant AIFA-2016-02364602), and E-Rare JTC 2016 grant ReDox.
Authorship
Contribution: M.T., A.F., Y.A., G.M., G.P., and F.L. conceived the study; M.T. and F.L. evaluated FSB staining; P. Milani, A.F., L.O., M. Basset, and G.P. performed clinical evaluations and acquired samples; M. Bozzola, G.F., and G.C. processed samples; L.V., P. Morbini, and M.P. performed and evaluated electron microscopy; G.P., M.N., and A.F. evaluated CR-PL; and M.T., M.U., K.O., F.L., and G.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanni Palladini, Centro per lo Studio e la Cura delle Amiloidosi Sistemiche, Fondazione IRCCS Policlinico San Matteo and University of Pavia, Viale Golgi 19, 27100 Pavia, Italy; e-mail: giovanni.palladini@unipv.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal