Abstract
Genetic predispositions to venous thromboembolism (VTE) are relatively frequent in the general population and comprise a heterogeneous group of disorders. Whereas the most frequent congenital risk factors for thrombosis only moderately increase the risk, a deficiency in antithrombin (AT), one of the most important natural inhibitors of blood coagulation, carries a higher risk. Congenital AT deficiency is an infrequently encountered genetic risk factor for VTE, and different subtypes vary with regard to their thrombotic risk. Patients with congenital AT deficiency, especially those with quantitative deficiency (type 1), may develop thrombosis early in life and often have a conspicuous family history of first- and second-degree relatives with VTE. Women are particularly affected because of the risk potentiation by combined estrogen/progestogen oral contraceptive use or pregnancy. The lack of controlled trials or even observational studies of large cohorts does not allow therapeutic decisions to be based on scientific evidence. In this review, we will discuss cases with thrombotic manifestations and the tailored management of patients with this congenital thrombosis risk factor.
Introduction
Antithrombin (AT; SERPINC1) is a natural anticoagulant that inactivates thrombin, factor Xa, and, to a lesser extent, other coagulation factors, such as factor IXa1-4 . The cofactors of AT are heparins that increase its enzymatic activity ≥1000-fold.5,6 AT deficiency was first described in 1965 by Olav Egeberg.7 Since then, thrombophilia, a term first coined by Nygaard and Brown8 in 1937 to describe excessive arterial thromboses, has become a huge area of research, especially in venous thromboembolism (VTE). Thrombophilia testing is now widely available and excessively used in daily routine, often not driven by scientific evidence. In recent years, thrombophilia testing, including testing for AT deficiency, has undergone a critical reappraisal.9 AT deficiency is infrequently observed, even in the patient population with VTE. There is large variability among AT-deficient patients with regard to genetic background, subtype, and clinical severity of subtype. In addition, other genetic predispositions to VTE, such as factor V Leiden10,11 or the prothrombin G20210A variation at the 3′ untranslated region,12 have turned out to be of limited importance with regard to prediction of first or recurrent thrombosis.13,14 However, patients with AT deficiency have a high risk of developing VTE and an increased recurrence rate compared with patients with mild thrombophilia.15,16 They may show a severe thrombotic tendency, including childhood thrombosis17 and pregnancy complications.17-19 Although no clear association between heterozygous AT deficiency and arterial thrombosis has been demonstrated,20,21 carriers of a homozygous AT mutation are at increased risk of arterial thrombosis.17,22 Hereditary AT deficiency is, in most cases, transmitted as an autosomal dominant disease with variable penetrance and equal distribution between sexes. Quantitative (type 1) and qualitative (type 2) AT defects are distinguished. In patients with type 1 defects, AT antigen level and activity are similarly reduced. In type 2 AT deficiency, antigen levels are normal, but the AT molecule is dysfunctional, and therefore, AT activity is reduced. Type 2 AT defects are differentiated into 3 variants, depending on the localization of the mutation: reactive site defects, in which the binding of thrombin to AT is impaired; heparin binding site (HBS) defects; and pleiotropic effect mutations, affecting the reactive site as well as the HBS.
Type 2 HBS defects are relatively frequent, at least in Europe, but associated with a low risk of VTE, except in cases of homozygosity, in which very severe thrombotic events and pregnancy complications are common.18,22-24 Type 1 AT deficiency is usually caused by nonsense or null mutations of SERPINC1 (which explains their phenotype) or defects in splicing.25 Type 2 deficiency is almost exclusively caused by missense mutations.1,26,27
The estimated prevalence of AT deficiency is ∼0.02% to 0.2% in the general population and ∼1% to 5% in patients with VTE.3,28-32 In patients with hereditary AT deficiency, ∼60% of VTE events occur spontaneously and 40% secondary to a transient risk factor.33,34 In retrospective analyses, ∼50% of patients with AT deficiency were found to have developed VTE by the age of 50 years.35-37
When we decide on diagnostic or therapeutic management, we usually rely on evidence from interventional studies. This is not possible in patients with AT deficiency. However, we have tried hard to base our suggestions on the limited data available in literature and on our own experience.
Case 1
Young woman with massive VTE during OC use
A 22-year-old woman was admitted with bilateral deep vein thrombosis (DVT) of the pelvic and femoral veins and pulmonary embolism (PE). She presented with dyspnea, which had been ongoing for 4 weeks, and massive swelling, discoloration, and pain in both legs, which had lasted for a few days. Her family history was quite conspicuous. Her paternal grandfather, granduncle, and aunt (postpartum) had died of thrombosis/PE. Her father had developed a life-threatening PE at 50 years of age after spine surgery and was on continuous anticoagulation. Another paternal aunt had also had recurrent thrombosis. Eight months before, the patient had started a combined estrogen/gestagen oral contraceptive (OC). The patient had a 17-year-old sister who had not experienced any thrombotic event.
First, she was put on low molecular weight heparin (LMWH). Interestingly, her anti-Xa level reached only therapeutic levels with 200 IU/kg body weight (BW) of LMWH twice daily. Antiphospholipid antibodies, factor V Leiden, and prothrombin G20210A variation were excluded. AT, protein C, and protein S were not determined at diagnosis. After 2 weeks of LMWH, she was put on rivaroxaban.
Three months after VTE diagnosis, she presented to the thrombosis and hemostasis outpatient unit of our hospital. While on 20 mg of rivaroxaban once daily, she had experienced heavy menstrual bleeding and was therefore switched to 2.5 mg of apixaban twice daily. She had continued swelling and pain in her right leg, compatible with postthrombotic syndrome. At that time, the thrombophilia testing was incomplete, so she was asked to quit apixaban for 3 days and was bridged with a prophylactic dose of LMWH. The thrombophilia screening revealed AT activity of 44% (normal range, 80%-120%). At repeated measurement, her AT activity was 54% 4 hours after 2.5 mg of apixaban, and AT antigen was 48%. She was advised to continue with 5 mg of apixaban twice daily. The genetic analysis showed a mutation of the SERPINC1 gene (exon 2: c.282_283insA, p.Tyr95IIfs*10 in heterozygous form), which corresponds to type 1 deficiency.
Comments on case 1
We do not perform testing for thrombophilia in most VTE patients.14,38 However, we think that in all patients with VTE, family history should be thoroughly recorded. On the basis of an individual’s history, including family history, we decide whether that patient should be further tested for the presence of AT deficiency or other thrombosis risk factors. We consider AT testing to be reasonable in patients with spontaneous VTE at a young age (<40 years), particularly in those with first-degree relatives with VTE or in young patients or children with multiple thromboses or thromboses at atypical sites. In our opinion, OC use or pregnancy at the time of VTE do not render thrombophilia testing unnecessary in young women with a positive family history. It is known that women with AT deficiency are at especially high risk of VTE during OC use39 and pregnancy.40
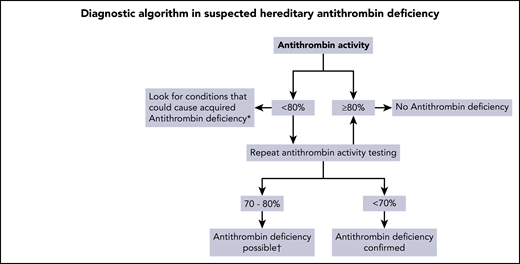
Functional AT activity assays are the mainstay of the diagnostic workup of hereditary AT deficiency. We have summarized a practical approach for the diagnostic workup of hereditary AT deficiency in Figure 1. In our experience and according to data from the literature, AT levels of patients with AT deficiency are usually clearly below the normal range (ie, <80%; most have levels <70%)17,41 ; however, patients with type 1 defects can also have levels up to 72% or even 78%.17,42 AT activity <70% is highly suggestive of AT deficiency, and a single measurement is sufficient to guide further treatment of VTE patients in the acute phase, particularly if the family history is positive. A confirmatory AT measurement should be performed a few weeks after the first measurement. In the case of a borderline AT activity level (70%-80%), genetic testing or confirmation of the deficiency in another family member (if not yet already available) should be considered, if possible. However, beyond determination of AT activity, further diagnostic workup does not seem to be mandatory, because it usually does not change treatment decisions. Further differentiation between the various types of AT deficiency is only possible in highly specialized and/or research laboratories; a diagnostic procedure has been proposed by Muszbeck et al.1 Table 1 lists the anticoagulant drugs for treatment of AT deficiency and their influence on AT determination. It had been suggested that warfarin might increase AT levels in patients with a deficiency, but in 2 reports, levels remained <80% of normal.43,44 A recent study evaluated AT levels in nondeficient patients who received warfarin; their AT levels were measured before and during treatment, and no change was found.45 Whether AT levels in AT-deficient patients on VKAs behave differently is not completely clear. It should be noted that during treatment with DOACs, levels of AT can be falsely high, and thus, the deficiency may be concealed.
Diagnostic algorithm in hereditary AT deficiency. *Causes of acquired AT deficiency: acute thrombosis, disseminated intravascular coagulation, liver disease, nephrotic syndrome, extracorporeal membrane oxygenation, hemodialysis, surgery or trauma, asparaginase therapy, heparin therapy, OC/estrogen use (modest). †Consider confirmation by genetic analysis or deficiency in another family member.
Diagnostic algorithm in hereditary AT deficiency. *Causes of acquired AT deficiency: acute thrombosis, disseminated intravascular coagulation, liver disease, nephrotic syndrome, extracorporeal membrane oxygenation, hemodialysis, surgery or trauma, asparaginase therapy, heparin therapy, OC/estrogen use (modest). †Consider confirmation by genetic analysis or deficiency in another family member.
Anticoagulants for prophylaxis and treatment of AT deficiency
| Class . | Drug . | Specific consideration . | |
|---|---|---|---|
| Diagnostic AT determination . | Prophylaxis and treatment* . | ||
| UFH | Decrease in AT during continuous infusion43 | Heparin resistance possible; if no adequate increase at usual UFH dose, increase dose or consider AT replacement; be aware of bleeding risk with high dose of UFH and AT replacement | |
| LMWH | Dalteparin, enoxaparin, tinzaparin, nadroparin, bemiparin | Decrease unlikely but cannot be excluded completely50 | Currently often used; seems effective by experience; dose has not been tested in clinical trials; anti-Xa measurement may not reflect anticoagulant effect but has been used |
| Indirect factor Xa inhibitor | Fondaparinux | No experience | Almost no data |
| VKA | Warfarin, acenocoumarol, phenprocoumon | All AT tests possible | Effective |
| DOAC | |||
| Thrombin inhibitor | Dabigatran | Falsely high levels if tested with FIIa-based assays80 | Most likely effective; no data |
| Factor Xa inhibitor | Apixaban, edoxaban, rivaroxaban | Falsely high levels if tested with FXa-based assays81 | Most likely effective; only rare cases |
| Class . | Drug . | Specific consideration . | |
|---|---|---|---|
| Diagnostic AT determination . | Prophylaxis and treatment* . | ||
| UFH | Decrease in AT during continuous infusion43 | Heparin resistance possible; if no adequate increase at usual UFH dose, increase dose or consider AT replacement; be aware of bleeding risk with high dose of UFH and AT replacement | |
| LMWH | Dalteparin, enoxaparin, tinzaparin, nadroparin, bemiparin | Decrease unlikely but cannot be excluded completely50 | Currently often used; seems effective by experience; dose has not been tested in clinical trials; anti-Xa measurement may not reflect anticoagulant effect but has been used |
| Indirect factor Xa inhibitor | Fondaparinux | No experience | Almost no data |
| VKA | Warfarin, acenocoumarol, phenprocoumon | All AT tests possible | Effective |
| DOAC | |||
| Thrombin inhibitor | Dabigatran | Falsely high levels if tested with FIIa-based assays80 | Most likely effective; no data |
| Factor Xa inhibitor | Apixaban, edoxaban, rivaroxaban | Falsely high levels if tested with FXa-based assays81 | Most likely effective; only rare cases |
DOAC, direct oral anticoagulant; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Literature on these considerations is cited in the text.
If genetic testing is possible, the detection of a mutation in SERPINC1 is confirmatory of AT deficiency and specifies the subtype diagnosis.46 Missense mutations seem to be associated with a lower risk of VTE compared with null mutations.27 This is the case for heterozygous type 2 HBS mutations, which are often due to a founder effect.47 Specific mutations might occur only regionally, such as a mutation with type 2 deficiency in Finland, which leads to an increased risk of juvenile stroke as well as pregnancy complications.48 In our institution, we perform genetic testing in cases with decreased AT activity levels, after exclusion of acquired AT deficiency conditions, to confirm the diagnosis of the deficiency and determine the subtype.
With regard to anticoagulant treatment in patients with AT deficiency, the drugs chosen and their dosages require careful consideration of various factors, which are summarized in Table 1. AT per se is a slow inhibitor of thrombin, factor Xa, and factor IXa, and only in the presence of heparin does it exhibit its full anticoagulant potential.49 In contrast, low AT plasma levels have an impact on the anticoagulant effect of heparins, including LMWH.
With regard to changes in AT level during treatment with UFH and LMWH, it has been observed that AT decreased during UFH treatment, but during LMWH treatment, AT remained virtually unchanged.50 This is important not only for diagnosis but also for treatment of patients with AT deficiency. It has been known for a long time that patients with AT deficiency may show heparin resistance, a situation in which patients require unusually high doses of UFH (>35 000 U per day) to achieve a therapeutic activated partial thromboplastin time.51 Heparin resistance can be eliminated by AT concentrate in patients undergoing cardiopulmonary bypass surgery,52 but similar comparative studies with AT replacement are not available for congenital AT deficiency. However, AT replacement might be considered in cases with laboratory heparin resistance or early recurrence or extension of thrombosis despite anticoagulation, which could be regarded as clinical heparin resistance.
Apart from the possibly lower efficacy of heparin and fondaparinux in patients with AT deficiency, measurement of anti-Xa activity levels also involves uncertainty. Many anti-Xa assays are commercially available, some with added AT. Others rely on the endogenous AT of the patient’s plasma.53 In a study of patients with AT deficiency and a control group with normal AT levels, Croles et al54 spiked the samples with UFH and LMWH, aiming to reach an anti-Xa activity of 0.8 IU/mL. Mean anti-Xa activity with LMWH in AT-deficient individuals, measured by an assay that did not contain exogenous AT, was only two-thirds of the activity in control individuals. The expected anti-Xa levels after LMWH spiking were found in all nondeficient participants but in only one-quarter of deficient participants. This study clearly demonstrates that reduced AT activity has an impact on anti-Xa levels, and thus, standard doses of LMWH may lead to undertreatment in AT-deficient individuals. For monitoring LMWH levels in patients with AT deficiency, we suggest using reagents without exogenous AT, because this might better reflect the anticoagulant potential.
For decades, VKAs have been used to treat patients with VTE, including those with AT deficiency, and it is commonly accepted that VKAs are effective anticoagulants for patients with AT deficiency. Can we use DOACs? We found only single case reports on the successful use of apixaban,55 edoxaban,56 or rivaroxaban.57 Therefore, clinical evidence for the use of DOACs in patients with AT deficiency is lacking. However, because DOACs act independently of AT, these drugs might gain importance in the treatment of acute VTE and as secondary prophylaxis in patients with AT deficiency. This is supported by experimental data from Fukuda et al58 showing that edoxaban was equally effective in wild-type mice and heterozygous AT-deficient mice in an inferior vena cava thrombosis model. We advised our patient to continue anticoagulation with 5 mg of apixaban twice daily. Whether 2.5 mg twice daily might be fully protective in AT deficiency as well, as shown in a general thrombosis population in the AMPLIFY-EXT study,59 is not known. We would reduce apixaban only if our patient’s menstrual bleeding worsened again.
Our patient’s family history was highly suggestive of hereditary thrombophilia. Because the diagnosis of AT deficiency has further impact on the expected recurrence rate and on management during pregnancy and peri- and postpartum, it is, in our opinion, important to either confirm or exclude such a diagnosis. Therefore, we supported thrombophilia testing, particularly determination of AT, in this patient.
Additional questions regarding the future management of our patient arose. First, should she be advised to continue anticoagulation, even though thrombosis occurred during OC use? Two facts led us to the decision to suggest prolonged anticoagulation: the likely elevated recurrence rate in AT-deficient patients and the severe and life-threatening manifestation. In a recent meta-analysis, the risks of first and recurrent thrombotic events were estimated.16 The annual recurrence risk was 8.8% in deficient vs 4.3% in nondeficient patients; the odds ratio for rate of recurrence was 2.1, with a 95% credible interval of 0.4 to 4.0. Differentiation between provoked and unprovoked events was not possible. Because there are no interventional trials on duration of anticoagulation in patients with AT deficiency available, each decision on long-term anticoagulation must be case based, considering the individual risk of recurrence of VTE and risk of bleeding. Second, advice on contraception is important for a young woman. There were no data to provide guidance for an informed decision. However, because of the high VTE risk during combined OC use,39,60 we did not suggest continuation but rather advocated for lower-risk options, such as intrauterine devices, including those with levonorgestrel, or progesterone-only pills.
Another important question involves the testing of family members. If the younger sister also had AT deficiency, her annual risk for a first VTE would be 1.2% according to a recent meta-analysis16 and thus clearly higher compared with a nondeficient individual (odds ratio, 14.0; 95% credible interval, 5.5-29.0). If the sister also used a combined OC, her annual risk of VTE would be between 2% and >10%.39,60 The absolute risk of pregnancy-associated VTE can be assumed to be as high as 16%.61 Therefore, we suggested informing the sister of the possibility of testing for AT deficiency. If the patient had had a brother, we would, in that particular family, also have advised testing after careful counseling. Although it has never been shown that testing improves outcome,62 and mortality currently is not increased in those with thrombophilia,63 we prefer a personalized approach in this infrequently encountered disease. The possible advantages are early diagnosis of VTE, as soon as symptoms of DVT or PE occur, and a more rigorous and tailored thrombosis prophylaxis in risk situations.33 However, it should be brought to the attention of family members that nondeficient individuals may also have an increased risk of thrombosis64 and that testing for thrombophilia might have a negative impact on insurance coverage.
Case 2
Thromboprophylaxis during surgery
Laparoscopic cholecystectomy was planned in a 35-year-old man. His medical history revealed that at the age of 19 years, he had developed a left-sided proximal DVT and concomitant bilateral segmental PE during an infection. AT antigen and activity levels, which had been measured in the acute phase and repeated twice thereafter, had consistently shown decreased AT antigen and activity values ranging between 40% and 50% (normal range, 80%-120%). The patient’s mother had developed PE after hip surgery and also had AT type 1 deficiency. After initial UFH treatment, VKAs had been initiated and continued without bleeding complications or VTE recurrence.
The patient was referred for the planning of perioperative management; the international normalized ratio (INR) was still 2.5, although VKAs had already been paused for 5 days. After administering 5 mg of vitamin K, the INR was 1.3 the next day, and a single dose of dalteparin at 100 IU/kg BW was administered in the morning. The following day, before the intervention, and the day after, 3000 IU of a plasma-derived AT concentrate were given, which was expected to raise AT levels to normal values (80%-120%; BW, 81 kg), and dalteparin was reduced to a prophylactic dose. On the second postoperative day, dalteparin was again raised to 100 IU/kg BW once daily, concomitantly with the reinitiation of a VKA, until the target INR was reached. No bleeding or VTE occurred.
Comments on case 2
No randomized controlled trials have been performed assessing the need for AT replacement or the dose of LMWH to be used in the perioperative setting in patients with hereditary AT deficiency. However, it has been shown that in patients with inherited thrombophilia, approximately half of all VTE events occur in transient risk situations.33 It seems reasonable to substitute AT when surgical procedures with an increased VTE risk are performed in patients with inherited deficiency and a personal and/or family history of thrombosis.3,65,66 In such situations, we suggest increasing AT to normal levels (80%-120%) and administering LMWH at the usual prophylactic dose, which is, in our opinion, safer with regard to bleeding than increasing the dose of LMWH. For the perioperative prevention of VTE, the US Food and Drug Administration has approved the use of plasma-derived and recombinant AT concentrates67 ; in Europe, only plasma-derived concentrates are available. If plasma-derived AT concentrates are used, bolus doses similar to that in our case can be applied. In a patient undergoing surgery with a very high thrombosis risk (eg, cancer surgery) or in women undergoing Cesarean section, we suggest a prolonged period of AT replacement (eg, 5 days) and regular measurements of AT trough levels, with an attempt to keep AT trough levels at least >70%. If recombinant AT is used, an initial bolus needs to be administered, followed by continuous infusion, because recombinant AT has a much shorter half-life (∼10 hours) than plasma-derived AT (∼2-3 days). A dosing schedule for AT replacement has been suggested by Bauer et al.66 In minor surgery with a low risk of VTE, we do not suggest AT replacement. In patients with uneventful personal and family histories, in those with heterozygous type 2 HBS AT deficiency, and in patients with only slightly decreased AT levels (eg, 70%-80%), we usually do not consider perioperative substitution with AT.
Case 3
Planning of thromboprophylaxis during pregnancy and delivery
A 31-year-old woman who had developed proximal DVT at age 21 years while using an OC was treated with a VKA for 6 months. She developed a recurrent VTE event at age 24 years, that time as unprovoked DVT, and symptomatic PE. Thrombophilia testing revealed an AT level of 56% (antigen level, 54%; normal range, 80%-120%), consistent with type 1 deficiency. Her family history was positive with regard to thrombotic manifestations; her mother had developed pregnancy-induced DVT and recurrence several years after the first VTE, and her sister experienced a first VTE after delivery and then also a recurrent DVT. In both, AT deficiency type 1 had been confirmed; furthermore, thrombosis in the maternal grandfather was reported. After the second event, the patient had been put on long-term anticoagulation with a VKA. Four years ago, she came to seek advice about her planned pregnancy. Counseling included information about an increased risk of VTE during pregnancy and after delivery, as well as the potential embryotoxic action of VKAs, especially when VKAs are not stopped before week 6.68 Because she had had 2 thrombotic events, the second unprovoked, she was kept on a VKA and was instructed by her gynecologist to closely observe her menstrual cycle and perform a pregnancy test as soon as her menstrual bleeding was delayed.
She recently became pregnant and stopped the VKA before the sixth week of gestation; her BW was 63 kg. She was immediately started on 150 IU/kg BW of enoxaparin once a day (peak anti-Xa, 0.48 IU/mL) by her gynecologist; at the 12th week of gestation, the dose was increased to 100 IU/kg twice daily (peak anti-Xa, 0.65 IU/mL; AT measurement with an assay without AT in the reagent). We provided a written recommendation to the obstetrician and the patient herself with statements regarding anticoagulation and AT replacement for the peri- and postpartum periods and a telephone number of the thrombosis and hemostasis 24/7 call service to manage unexpected situations. AT replacement around the time of delivery will start with a dose that will lead to an increase in AT levels up to the normal range (50 U/kg BW), and we plan to keep trough levels at least at 70% within the first 5 days after delivery. During that time, only prophylactic LMWH will be given. On day 2 after delivery, a VKA, either warfarin or acenocoumarol,69 will be reinitiated in parallel with LMWH and AT replacement, as long as there is no increased postpartum bleeding. In case of Cesarean section, we will start AT replacement before surgery. AT replacement is not a contraindication to epidural anesthesia; however, the time interval to last administration of LMWH must be considered. We stop prophylactic anticoaguation 12 hours and therapeutic anticoagulation 24 hours before Cesarean section and give the first prophylactic dose of LMWH as soon as possible ≥4 hours after epidural-catheter removal.70
Comments on case 3
Pregnant women with inherited AT deficiency are at high risk of developing severe thromboembolic complications, and rates of fetal loss and stillbirth are increased.34,71-74 Because of the high risk of VTE, it is advisable to initiate thromboprophylaxis during pregnancy.61,69 We start early, bearing in mind that VTE frequently occurs in the first trimester.75 With regard to dose, we agree with the Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology2 and a multidisciplinary group74 that prophylactic doses may not be enough; therapeutic doses are probably needed in AT-deficient patients. This is also in accordance with the standard protocol applied by Bramham et al.76 Substitution of AT should be considered in the peri- and postpartum periods, when high doses of LMWH might increase the bleeding risk. In our institution, we usually use AT concentrates in AT-deficient patients with type 1 defects, type 2 reactive site defects, and a personal or family history of VTE and in homozygous type 2 HBS women. If the subtype is unavailable, decisions in favor of replacement can be based on AT level (<60%), previous positive history of thrombosis, or positive family history of thrombosis.74
Case 4
Patient with unprovoked VTE during adolescence
A 13-year-old girl presented with pain and swelling in the right leg for 4 days and fever for 2 days. Three weeks earlier, she had been bedridden for 3 days because of a flu-like infection. She had no other previous illnesses, and her family history revealed no VTE. Duplex sonography showed proximal thrombosis from the femoral up to the inferior cava vein. In a computed tomography scan, additional thromboses of the portal and superior mesenteric veins and multiple collaterals were found. Laboratory tests revealed an AT activity of only 14%, showing a severe deficiency in AT, the extent of which could not be explained by consumption of AT from thrombosis. She was born in Vienna, Austria, and of Roma origin; her parents had been born in Serbia.
Treatment was initiated by raising AT values through replacement (target value, 80% AT activity) and starting continuous UFH. After 3 days, UFH was switched to therapeutic doses of LMWH. Treatment with a VKA was started, and AT substitution and LMWH were stopped as soon as the INR was within therapeutic range. Under this treatment, the swelling of her right leg almost completely regressed, and imaging of the portal and mesenteric veins showed almost complete resolution of thrombi.
Results of genetic testing revealed the presence of homozygous type 2 HBS AT deficiency, also called AT Budapest 377 (c.391C>T p.Leu131Phe). AT antigen was 72%. Both parents and a brother were heterozygous carriers. She was not continuously compliant with the treatment, and after 8 years, she developed a proximal DVT of the left leg (INR subtherapeutic). At that time, the target INR was increased to 2.5 to 3.5, but anticoagulation was soon switched to 5 mg of apixaban twice daily, and she has been managed without complications since then.
Comments on case 4
AT type 2 HBS defects are the most common but least prothrombotic of AT mutations. Consistently, the parents of our patient had never had a VTE event. However, because of homozygosity, our patient developed VTE at an early age during a minor-risk situation.78 Immediate diagnosis of AT deficiency and normalization of AT levels together with therapeutic anticoagulation led to rapid improvement. Potential doubts regarding the switch of anticoagulation from a VKA to apixaban after recurrence may arise as a result of the fundamental lack of clinical data supporting the use of DOACs in patients with AT deficiency. However, our decision was based on logical considerations that are discussed in case 1.
It must be mentioned that homozygous AT deficiency type 2 HBS may lead to very severe venous and arterial thrombotic events (eg, massive stroke) in newborns and children; therefore, early diagnosis and treatment are necessary.22,79 According to the literature, most patients with type 2 HBS originate from the Balkan region, and we know about the founder effect of the Budapest 3 mutation.47 In most cases, their functional AT level is <35%.18,19 These women also have an exceptionally high risk of miscarriage and fetal death. Successful pregnancy outcomes are rare and have only been observed in women on anticoagulation. Even in women with concomitant LMWH and AT replacement during pregnancy, the risk of pregnancy loss was 50%.18,19
Discussion
Hereditary AT deficiency is an infrequently encountered but strong risk factor for VTE, except in patients with heterozygous type 2 HBS deficiency. We think that patients with congenital AT deficiency should be advised and managed differently from individuals with more frequent but less strong genetic thrombosis risk factors, such as factor V Leiden, or those without thrombophilia. We are convinced that affected patients should be identified. Further evaluation of the management of such patients and their families should be performed in specialized thrombosis and hemostasis centers. Although AT deficiency is distributed equally among women and men, women are particularly affected because of the risk potentiation by OC use or pregnancy. Making carriers aware of their increased risk and counseling them on an individualized basis, discouraging them from taking combined estrogen/progestogen contraceptives, prescribing long-term anticoagulation in those with previous VTE, and providing specific care for pregnant women are, in our opinion, the mainstays of the management of patients with hereditary AT deficiency.
Acknowledgments
The authors thank Tanja Altreiter (Department of Medicine I, Medical University of Vienna) for proofreading this manuscript.
The Medical University of Vienna received an unrestricted grant from CSL Behring.
Authorship
Contribution: I.P. and J.T. performed the literature research, wrote the manuscript, and approved the final version.
Conflict-of-interest disclosure: I.P. occasionally receives honoraria for lectures or advisory board meetings from Bayer, Boehringer Ingelheim, CSL Behring, Pfizer, and Daiichi Sankyo. J.T. declares no competing financial interests.
Correspondence: Ingrid Pabinger, Clinical Division of Hematology and Hemostaseology, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: ingrid.pabinger@meduniwien.ac.at.