In this issue of Blood, Prevel et al demonstrate that older adults (>60 years) with immune thrombotic thrombocytopenic purpura (iTTP) have a distinct presentation and outcomes compared with younger adults.1 Atypical neurologic symptoms, such as headache, confusion, and focal deficits, are common, and anemia and thrombocytopenia are less prominent, leading to delays in diagnosis and therapy. Mortality at 1 month and 1 year was higher in older adults, who also had inferior long-term survival compared with older adults without iTTP.
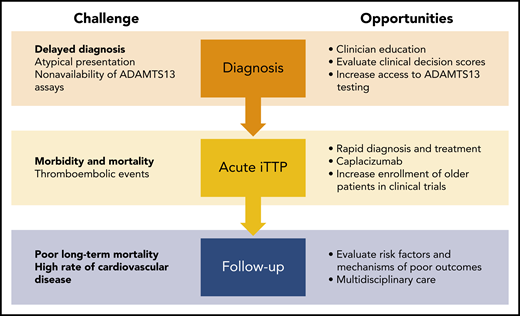
Challenges and opportunities to improve outcomes for adults with iTTP. Atypical presentations often lead to delayed diagnosis in older adults. Clinician education and improved access to diagnostic assays may reduce time to diagnosis and treatment. Thromboembolic events are the leading cause of death during acute TTP and cause significant morbidity from end-organ injury such as myocardial infarction and stroke. Using caplacizumab during acute TTP may reduce ischemic events and shorten the duration of plasma exchange, which will also reduce the risk for catheter-related complications that were common in older adults. Finally, additional research is needed to identify risk factors for worse long-term outcomes after TTP. Because cardiovascular disease (rather than TTP relapse) is associated with worse long-term survival, a multidisciplinary approach is recommended to improve the care of older adults with TTP.
Challenges and opportunities to improve outcomes for adults with iTTP. Atypical presentations often lead to delayed diagnosis in older adults. Clinician education and improved access to diagnostic assays may reduce time to diagnosis and treatment. Thromboembolic events are the leading cause of death during acute TTP and cause significant morbidity from end-organ injury such as myocardial infarction and stroke. Using caplacizumab during acute TTP may reduce ischemic events and shorten the duration of plasma exchange, which will also reduce the risk for catheter-related complications that were common in older adults. Finally, additional research is needed to identify risk factors for worse long-term outcomes after TTP. Because cardiovascular disease (rather than TTP relapse) is associated with worse long-term survival, a multidisciplinary approach is recommended to improve the care of older adults with TTP.
Over the past 30 years, the advent of plasma exchange and effective immunosuppression has reduced mortality of acute iTTP from >90% to <10%; however, iTTP outcomes in older adults are less encouraging with >25% dying during the acute episode.2 Factors contributing to increased short-term mortality in older adults are unclear. For the first time, Prevel and colleagues present data to suggest that delays in diagnosis may contribute to poorer outcomes in older adults. They found that time to iTTP diagnosis was 2 days longer for patients >60 years. This delay was largely driven by atypical and nonspecific presenting features, including variable neurologic abnormalities, such as delirium, behavioral changes, or transient ischemic attacks and stroke, which are common in older adults. In addition, iTTP diagnostic scores such as the French Index, have lower sensitivity (60%) in older adults, who commonly present with greater renal impairment and less profound thrombocytopenia than their younger counterparts. This begs the sobering conclusion that a number of older adults with iTTP may never be correctly diagnosed.
Most deaths during acute iTTP result from ischemic events such as myocardial infarction and stroke, which occurred at a several-fold higher rate in older adults. Older patients have higher rates of preexisting cardiovascular morbidities, such as hypertension and coronary artery disease, suggesting that these patients with senescent endothelium may have diminished vascular reserve and a lower threshold to develop ischemic injury. Caplacizumab, a nanobody that targets the von Willebrand factor–platelet interaction and inhibits microvascular thrombosis, was recently approved for patients with acute iTTP and is particularly attractive in this setting because it reduced the rate of major thromboembolic events in clinical trials. However, bleeding is the major adverse effect of caplacizumab, and this may be of greater concern in older patients, who are more likely to have bleeding risk factors, such as concomitant anticoagulant or antiplatelet therapy.
Prevel et al also found that older patients who survived their acute iTTP episode had threefold higher long-term mortality compared with an age-matched reference population from the same geographic area. This is consistent with findings from the Oklahoma Thrombotic Thrombocytopenic Purpura (TTP) Registry that showed reduced survival in TTP patients of all ages.3 The authors hypothesized that shortened survival could be attributed to coexisting disorders, such as hypertension, depression, and cognitive decline. Interestingly, in the Oklahoma registry, the majority (67%) of deaths was due to cardiac causes or stroke rather than TTP relapse. Although specific causes of death were not reported in the current study, iTTP survivors had higher rates of stroke and coronary artery disease than the control population. Ischemic injury during acute iTTP or worsening of chronic organ impairment during iTTP can account for some of this burden. However, cardiovascular events may be more common after recovery from iTTP.4 Indeed, a recent study showed that stroke after iTTP is associated with persistently low ADAMTS13 activity during iTTP remission independent of age.5 Previously, large population-based studies have demonstrated an association between lower ADAMTS13 activity with myocardial infarction and stroke.6,7 Remission ADAMTS13 activity after recovery from acute iTTP is an attractive candidate as a biomarker and therapeutic target to improve long-term outcomes in all patients with iTTP.
In the United States alone, the population aged ≥65 years will nearly double in the next 40 years,8 from 56 million in 2020 to 94.7 million in 2060, and the population >80 years is growing even faster.8 An increase in older patients with hematologic disorders is inevitable and has led to the burgeoning field of geriatric hematology. Aging is associated with numerous and diverse alterations in cellular function coupled with reduced function of vital organs.9 Aging-associated vulnerabilities, including impaired cognitive and functional status, comorbidities, and polypharmacy, uniquely impact disease phenotypes, response to therapy, and survival. This phenomenon is not restricted to iTTP and is observed across the spectrum of hematologic (and other) disorders. However, older adults are underrepresented in clinical trials.10 For example, patients enrolled in the phase 3 trial of caplacizumab in iTTP (HERCULES) were relatively young, with a median age of 46 years, and it is challenging to extrapolate efficacy and safety data to older individuals. To improve outcomes in this vulnerable population, it is our responsibility to advocate for the inclusion of older individuals and for rigorous assessment of comorbidities in clinical trials to provide data directly applicable to an aging patient population.
How can we improve outcomes for older patients with iTTP (see figure)? Delayed diagnosis presents the first opportunity for intervention. Because ADAMTS13 testing is still not immediately available at most centers, clinicians use decision support tools, such as the French TTP Index and PLASMIC score; these need to be evaluated in and potentially modified for older patients. Increasing awareness of neurologic symptoms as presenting features of iTTP will require education of internists, emergency physicians, and neurologists, who are often the first point of contact. Additional work is also needed to establish the optimal treatment of iTTP in older adults. Enrollment and stratified analysis of the elderly in clinical trials are paramount for understanding efficacy and tolerability in this vulnerable subgroup. Finally, long-term follow-up of older adults who survive an acute episode of iTTP is an area ripe for investigation and improvement. Because cardiovascular disease, cognitive impairment, and reduced functional status are common after iTTP recovery in older adults, patients are likely to benefit from multidisciplinary management. Moreover, identification of novel therapeutic targets, including ADAMTS13, may pave the way, not only for prognosticating adverse outcomes7 but also for developing treatments to ameliorate the long-term course of the disease.
Conflict-of-interest disclosure: S.C. has served as a consultant for Alexion and Sanofi-Genzyme and has received research support from Shire through a grant from the Hemostasis and Thrombosis Research Society. A.C. has served as a consultant for Synergy, and his institution has received research support on his behalf from Alexion, Bayer, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda.