TO THE EDITOR:
Essential thrombocythemia (ET) is a myeloproliferative neoplasm (MPN) defined by thrombocytosis, increased risk of vascular thrombosis,1,2 hemorrhage,3 and progression to myelofibrosis4,5 and acute myeloid leukemia.4,5 Patients are risk-stratified to identify those who might benefit from cytoreduction to reduce the risk of vascular complications.6 Resistance/intolerance to hydroxycarbamide (HC-RES/INT), a first-line cytoreductive treatment, develops in 20% of high-risk patients7 with increased risk of disease progression and reduced survival.8 New approaches are needed to predict disease-transformation risk in these patients, together with development of therapies that reduce this risk.
Following the discovery of the Janus kinase 2 (JAK2) mutation (JAK2V617F), present in ∼50% of ET,9 the first approved JAK1/JAK2 inhibitor, ruxolitinib (RUX), is now widely used for treatment of myelofibrosis10 and polycythemia vera.11 The MAJIC-ET trial explored the role of RUX in HC-RES/INT ET, randomizing patients 1:1 to RUX or best available therapy (BAT), demonstrating similar rates of 1-year complete hematological response.12 Mutational status was not comprehensively reported in this paper. This is important as ET patients (29% to 52.5%)13,14 carry mutations in non-MPN driver genes (NDMs). Inferior prognosis is associated with specific mutations at diagnosis.14 The impact of NDMs in HC-RES/INT ET is unknown, as is the effect of RUX on disease course in molecularly defined subgroups. We therefore evaluated the mutational status of MAJIC-ET patients and correlated this with clinical outcomes.
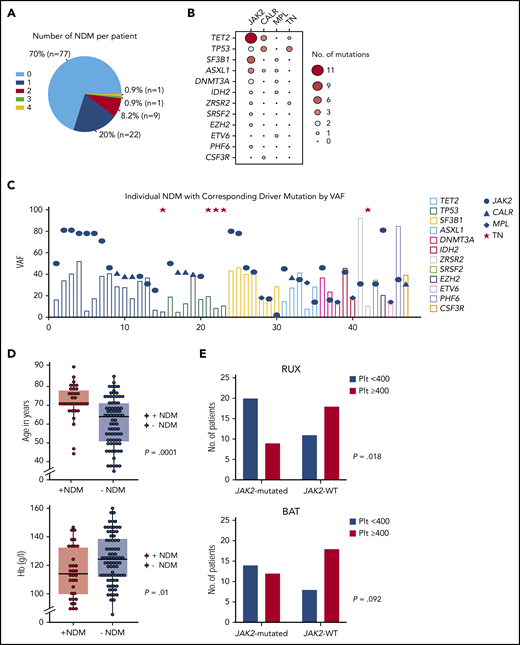
Next-generation sequencing was performed at baseline (n = 110) and serially if a later sample was available (see supplemental Methods [available on the Blood Web site] for next-generation sequencing and statistical analysis methodology). Median follow-up was 55 months (95% confidence interval [CI], 49.9-60.4). JAK2, CALR, and MPL mutations were present in 49.1%, 30%, and 4.5% of patients, respectively, and 16.4% of patients were “triple negative” (TN). Baseline NDMs were present in 30% (n = 33) of patients with >1 present in 10% (Figure 1A), most frequently TET2 (n = 12), TP53 (n = 7), and SF3B1 (n = 7) genes (Figure 1B; supplemental Table 1). Driver mutation variant allele frequency (VAF) was higher than NDM VAF in 66.67%, 87.5%, and 20% of JAK2, CALR, and MPL-mutated patients, respectively (Figure 1C). Patients with NDMs tended to be older with lower hemoglobin levels (Figure 1D; supplemental Table 2). TP53 mutations trended toward a higher frequency in TN (17.6%) than in JAK2/CALR/MPL-mutated patients (4.3%; P = .073). In the primary analysis, driver mutation status did not correlate with CHR.12 Since platelet count reduction is a key therapeutic goal, we performed a post hoc analysis defining platelet response as <400 × 109/L at 1 year. RUX-treated JAK2V617F-mutated patients had significantly more platelet responses than JAK2V617F wild-type (WT) patients, a difference not seen for BAT-treated patients (Figure 1E). RUX discontinuation more often occurred in non-JAK2V617F–mutated patients (odds ratio, 3.9; 95% CI, 1.2% to 13.1%; P = .027) in whom treatment failure was the most frequent cause (41.7%, n = 10 of 24) followed by treatment toxicity (33.3%; n = 8 of 24). In contrast, in JAK2V617F-mutated patients, the commonest cause for RUX discontinuation was a transformation event (43.8%; n = 7 of 16) followed by treatment failure (31.3%; n = 5 of 16). NDMs did not influence hematological/symptom responses (supplemental Table 3).
Baseline mutational analysis and correlation with clinical characteristics and treatment response. (A) Pie chart showing number of NDMs per patient. (B) Balloon plot showing association of driver mutations with NDMs with size and color of bubble corresponding to frequency of association; NDMs were more often associated with JAK2V617F mutations. (C) Column and dot plot showing variant allele frequencies (VAFs) of each NDM (column) with corresponding driver mutation (blue dot/triangle/diamond). Red star indicates patient with TN. Driver mutation VAF was higher in 66.67%, 87.5%, and 20% of JAK2-, CALR-, and MPL-mutated patients suggesting driver mutation acquisition first in these, although with the caveat that order of mutation acquisition can only be definitively assigned using single-cell methodologies.23 (D) Dot and box plots of age at trial entry in patients with NDMs compared with patients without NDMs; median age 71 vs 64 years, P = .0001 (top plot). Hemoglobin (Hb) level (mean Hb, 115 g/L) was lower in patients with NDMs compared with patients without NDMs (mean Hb, 125 g/L), P = .01 (bottom plot). Dots represent each individual patient and each horizontal line and box represent the median for age/mean for Hb and interquartile ranges, respectively, using the Mann-Whitney U test to compare median ages (non-normal distribution) and the Student t test to compare Hb means (normal distribution). (E) Post hoc analysis of 1-year platelet count responses; significantly more patients on RUX who were JAK2-mutated achieved platelet count (plt) <400 than non-JAK2–mutated patients (top bar chart). This difference was not seen within the BAT arm (bottom bar chart). JAK2, JAK2V617F; Plt < 400, platelet count of <400 × 109/L; Plt ≥ 400, platelet count of ≥400 × 109/L; TN, triple negative; WT, wild type.
Baseline mutational analysis and correlation with clinical characteristics and treatment response. (A) Pie chart showing number of NDMs per patient. (B) Balloon plot showing association of driver mutations with NDMs with size and color of bubble corresponding to frequency of association; NDMs were more often associated with JAK2V617F mutations. (C) Column and dot plot showing variant allele frequencies (VAFs) of each NDM (column) with corresponding driver mutation (blue dot/triangle/diamond). Red star indicates patient with TN. Driver mutation VAF was higher in 66.67%, 87.5%, and 20% of JAK2-, CALR-, and MPL-mutated patients suggesting driver mutation acquisition first in these, although with the caveat that order of mutation acquisition can only be definitively assigned using single-cell methodologies.23 (D) Dot and box plots of age at trial entry in patients with NDMs compared with patients without NDMs; median age 71 vs 64 years, P = .0001 (top plot). Hemoglobin (Hb) level (mean Hb, 115 g/L) was lower in patients with NDMs compared with patients without NDMs (mean Hb, 125 g/L), P = .01 (bottom plot). Dots represent each individual patient and each horizontal line and box represent the median for age/mean for Hb and interquartile ranges, respectively, using the Mann-Whitney U test to compare median ages (non-normal distribution) and the Student t test to compare Hb means (normal distribution). (E) Post hoc analysis of 1-year platelet count responses; significantly more patients on RUX who were JAK2-mutated achieved platelet count (plt) <400 than non-JAK2–mutated patients (top bar chart). This difference was not seen within the BAT arm (bottom bar chart). JAK2, JAK2V617F; Plt < 400, platelet count of <400 × 109/L; Plt ≥ 400, platelet count of ≥400 × 109/L; TN, triple negative; WT, wild type.
Transformation events occurred in 12.7% (supplemental Table 3). TP53-mutated patients had inferior 4-year transformation-free survival (TFS) of 42.9% (95% CI, 9.8% to 73.4%) vs 79.8% (95% CI, 69.7% to 86.8%) for WT patients (P = .011) (Figure 2A). Splicing factor (SF) mutations conferred a poorer 4-year TFS of 40% (95% CI, 12.3% to 67%) vs 81.5% for WT patients (95% CI, 71.4% to 88.3%; P = .00039) (Figure 2B), predominantly attributable to mutated SF3B1 (P = .004). High molecular risk (HMR) mutations in this cohort (defined by SF and TP53 mutations) conferred poorer TFS (P < .0001) (Figure 2C), which was not ameliorated by RUX (Figure 2D). HMR mutations retained their negative impact on multivariable analysis (Figure 2E). Driver mutation VAF ≥50% and male sex independently conferred poorer TFS, findings reported by other groups.15,16 Mutated TET2 did not correlate with clinical outcomes, comparable to previous findings.14
Kaplan-Meier curves of TFS stratified by mutational statuses with 4-year survival estimates. (A) TP53 mutations were associated with inferior 4-year TFS; TP53-mutated (42.9% [95% CI, 9.8% to 73.4%]) vs TP53-WT patients (79.8% [95% CI, 69.7% to 86.8%]) (P = .011). (B) SF mutations conferred a poorer 4-year TFS; SF-mutated (40% [95% CI, 12.3% to 67%]) vs SF-WT (81.5% [95% CI, 71.4% to 88.3%]) (P = .00039). (C) Comparing TFS in patients with HMR with low molecular risk (LMR) at 4 years; HMR 41.2% (95% CI, 23.3% to 72.7%) vs LMR 84.6% (95% CI, 76.9% to 93.1%) (P < .0001). (D) Stratifying patients with HMR mutations in this study by treatment arm demonstrates no amelioration of the negative impact of HMR mutation with RUX treatment; patients with HMR on RUX had TFS at 4 years of 36.4% (95% CI, 26.2% to 46.6%) and on BAT 50% (29.1% to 67.7%) (P = .505 between these arms) as compared to those without these mutations (ie, LMR) had TFS at 4 years of 84.7% (95% CI, 71.6% to 92%) on RUX and of 90.6% (95% CI, 78.5% to 96%) on BAT (P = .101 between these arms). The log-rank test was used to compare survival estimates between groups. (E) Forest plot showing multivariable Cox model of TFS. Covariates significant on univariate analysis were included; TP53 mutations, SF mutations, treatment arm, JAK2V617F mutation status, disease duration at trial entry (TE), age, and sex. HMR mutations independently retained negative impact on TFS with a hazard ratio (HR) of 4.21 (P = .006). Treatment arm, JAK2V617F status, disease duration at TE, and age were not significant but, notably, male sex was associated with a poorer TFS (HR, 4.5; P = .006). Driver mutation allele ≥50% was independently associated with a poorer TFS (HR, 4.11; P = .016). Age and disease duration at TE were categorized as continuous variables. Significant P values on forest plot; *P < .05, **P < .01. HMR, high molecular risk (SF and TP53 mutations); JAK2, JAK2V617F; LMR, low molecular risk (without SF or TP53 mutations); SF, splicing factor mutation (SF3B1, ZRSR2, SRSF2).
Kaplan-Meier curves of TFS stratified by mutational statuses with 4-year survival estimates. (A) TP53 mutations were associated with inferior 4-year TFS; TP53-mutated (42.9% [95% CI, 9.8% to 73.4%]) vs TP53-WT patients (79.8% [95% CI, 69.7% to 86.8%]) (P = .011). (B) SF mutations conferred a poorer 4-year TFS; SF-mutated (40% [95% CI, 12.3% to 67%]) vs SF-WT (81.5% [95% CI, 71.4% to 88.3%]) (P = .00039). (C) Comparing TFS in patients with HMR with low molecular risk (LMR) at 4 years; HMR 41.2% (95% CI, 23.3% to 72.7%) vs LMR 84.6% (95% CI, 76.9% to 93.1%) (P < .0001). (D) Stratifying patients with HMR mutations in this study by treatment arm demonstrates no amelioration of the negative impact of HMR mutation with RUX treatment; patients with HMR on RUX had TFS at 4 years of 36.4% (95% CI, 26.2% to 46.6%) and on BAT 50% (29.1% to 67.7%) (P = .505 between these arms) as compared to those without these mutations (ie, LMR) had TFS at 4 years of 84.7% (95% CI, 71.6% to 92%) on RUX and of 90.6% (95% CI, 78.5% to 96%) on BAT (P = .101 between these arms). The log-rank test was used to compare survival estimates between groups. (E) Forest plot showing multivariable Cox model of TFS. Covariates significant on univariate analysis were included; TP53 mutations, SF mutations, treatment arm, JAK2V617F mutation status, disease duration at trial entry (TE), age, and sex. HMR mutations independently retained negative impact on TFS with a hazard ratio (HR) of 4.21 (P = .006). Treatment arm, JAK2V617F status, disease duration at TE, and age were not significant but, notably, male sex was associated with a poorer TFS (HR, 4.5; P = .006). Driver mutation allele ≥50% was independently associated with a poorer TFS (HR, 4.11; P = .016). Age and disease duration at TE were categorized as continuous variables. Significant P values on forest plot; *P < .05, **P < .01. HMR, high molecular risk (SF and TP53 mutations); JAK2, JAK2V617F; LMR, low molecular risk (without SF or TP53 mutations); SF, splicing factor mutation (SF3B1, ZRSR2, SRSF2).
Thrombotic events (19.1%, n = 21 of 110) were not influenced by mutational status overall. This is in contrast to previous studies reporting a greater thrombotic risk in JAK2V617F-mutated patients.4 A possible explanation is that this association is not seen in HC-RES/INT patients who have a longer disease course and have undergone treatment, often with multiple lines of therapy. Furthermore, the number of events here is small and should therefore be interpreted with caution. Hemorrhagic events (9.1%; n = 10 of 110) were specifically associated with SF mutations (P = .007) (supplemental Table 3). Grade 3/4 hematological toxicities were not associated with mutational status. Overall survival at 4 years was 91.5% (95% CI, 80.2% to 96.4%) in BAT and 83% (95% CI, 70.4% to 90.5%) in RUX arms (P = .22) and was not influenced by mutational status.
One-year driver mutation molecular responses (MRs) were rare (n = 3), occurring exclusively in the RUX arm: a complete MR in 2 patients (JAK2V617F-mutated and CALR-mutated) and 1 CALR-mutated partial MR. Longitudinal driver mutation analysis was performed in 54% (n = 50 of 93); median analysis time was 48 months (24-60 months) with no significant change in VAF at any time point (supplemental Figure 1A-B). One-year MR was subsequently lost in 2 patients (supplemental Figure 1C-D) in association with clonal evolution of NDMs in both cases. Longitudinal NDM analysis was possible in 52% (n = 57 of 110); median analysis time was 40 months (6-60 months). New NDMs, defined by identification at VAF ≥5%, were detected in 19.3% (n = 11 of 57) at a similar frequency across treatment arms (supplemental Table 4) and no significant correlations were detected with baseline NDMs or clinical/survival outcomes. However, a median follow-up time of 10.7 months (95% CI, 9.05-12.4) after later NDM analysis is not sufficient time for survival analysis. These data highlight the clinical utility of serial molecular analysis in HC-RES/INT ET.
In this analysis, we identify NDMs at baseline in 30% of patients with ET, a higher frequency than most previous analyses, which may relate to the high-risk nature of this cohort.13-15,17 TP53 and SF3B1 mutations were observed, each at 6.4%, higher than previously reported in ET (∼2% and 2% to 5%, respectively).13-15,18 This may relate to the fact that this study analyzes a particular high-risk cohort for which there are limited data published on mutation profiles for comparison. The frequent detection of TP53 mutations in TN patients was unexpected but the numbers are too few (n = 3) to draw firm conclusions. Disease transformation was specifically associated with SF (most commonly SF3B1) and TP53 mutations, determining HMR for this cohort. Although prevalence of non-SF3B1 SF mutations in this cohort was low, we included these as HMR as they are established adverse-risk mutations in MPNs.15 However, this definition of HMR requires independent validation in larger cohorts before being applied in clinical practice. TP53 mutations in MPNs have been associated with acute myeloid leukemia transformation14,15 but have not been reported to increase myelofibrotic transformation in ET.14,15 Myelofibrotic transformation has been reported in association with SF mutations in ET, most often mutated SF3B1,14,19 but a recent large MPN study identified SRSF2, ZRSR2, and U2AF1 but not SF3B115 as myelofibrotic transformation predictors in ET. This contrasts with myelodysplastic syndromes in which SF3B1 mutations confer better survival20-22 with lower risk of disease progression,20 suggesting that disease context and comutations (primarily JAK2V617F here) are relevant.
Importantly, disease transformation in HMR patients was not mitigated by RUX, which is noteworthy as there has been interest in the possibility that early intervention with JAK2 inhibition might attenuate disease progression. We observed a novel association between SF mutations and hemorrhagic events; this finding needs independent corroboration due to low event rate. We also found that JAK2V617F-mutated status correlated with improved platelet responses to RUX, and, notably, more non-JAK2V617F–mutated patients stopped RUX, raising the possibility that JAK2V617F-mutated ET patients might selectively benefit from RUX.
In summary, we report for the first time, comprehensive mutational analysis of HC-RES/INT ET within the context of a prospective randomized clinical trial. We found a particularly high prevalence of TP53 and splicing factor mutations, which were strongly predictive of subsequent disease transformation, and not mitigated by RUX. This highlights the clinical/prognostic utility of serial mutation screening in HC RES/INT ET to allow identification of patients at risk of disease transformation.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank all of the patients who participated in this study, as well as the principal investigators and their teams for contributing to the trial.
This trial was supported by Bloodwise under the Trials Acceleration Program. This work was supported by a Medical Research Council Senior Clinical Fellowship (A.J.M., MR/l006340/1) and a Cancer Research UK (CRUK) Senior Cancer Research Fellowship, a Medical Research Council (MRC) Molecular Hematology Unit Core Award (MC_UU_12009/5; A.J.M.), and an MRC Clinical Research Training Fellowship (MR/S001190/1; J.M.O.). C.Y. was funded by grant C22436/A25354 from CRUK. This research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC).
The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR, the Department of Health, or the US National Institutes of Health (NIH).
Authorship
Contribution: J.M.O. analyzed experiments, performed the statistical analysis, and wrote the manuscript; A.H. oversaw the myeloid gene panel analysis and contributed to the writing of the manuscript; R.B. and A.P. were involved in the statistical analysis with senior oversight from C.Y.; S.A. and S.F. contributed to data collection; H.D., K.H., and P.W. processed samples, performed experiments, and analyzed data; N.C.P.C. analyzed experiments; M.F.M. contributed to data analysis; C.N.H. conceived and supervised the project and contributed to the writing of the manuscript; A.J.M. conceived and supervised the project, designed and analyzed experiments, and wrote the manuscript; and all authors read and approved the submitted manuscript.
Conflict-of-interest disclosure: Novartis provided an educational grant to support the trial and provided ruxolitinib free of charge. A.J.M. has participated in advisory boards for Novartis, CTI, and Baxaltra; received honoraria from Novartis, Gilead, Shire, and Baxaltra; and also received research funding and travel, accommodation, and expenses from Novartis. A.H. has participated in advisory boards for Novartis, and received honoraria from Gilead, Pfizer, and Roche. N.C.P.C. received honoraria for Novartis, Pfizer, and Incyte. M.F.M. received honoraria from Novartis and Celgene. C.Y. received honoraria from Celgene. The remaining authors declare no competing financial interests.
The current affiliation for C.Y. is Clinical Trials and Statistics Unit, The Institute of Cancer Research, London, United Kingdom.
Correspondence: Adam J. Mead, MRC Molecular Hematology Unit, MRC Weatherall Institute of Molecular Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford OX3 9DS, United Kingdom; e-mail address: adam.mead@imm.ox.ac.uk.

![Kaplan-Meier curves of TFS stratified by mutational statuses with 4-year survival estimates. (A) TP53 mutations were associated with inferior 4-year TFS; TP53-mutated (42.9% [95% CI, 9.8% to 73.4%]) vs TP53-WT patients (79.8% [95% CI, 69.7% to 86.8%]) (P = .011). (B) SF mutations conferred a poorer 4-year TFS; SF-mutated (40% [95% CI, 12.3% to 67%]) vs SF-WT (81.5% [95% CI, 71.4% to 88.3%]) (P = .00039). (C) Comparing TFS in patients with HMR with low molecular risk (LMR) at 4 years; HMR 41.2% (95% CI, 23.3% to 72.7%) vs LMR 84.6% (95% CI, 76.9% to 93.1%) (P < .0001). (D) Stratifying patients with HMR mutations in this study by treatment arm demonstrates no amelioration of the negative impact of HMR mutation with RUX treatment; patients with HMR on RUX had TFS at 4 years of 36.4% (95% CI, 26.2% to 46.6%) and on BAT 50% (29.1% to 67.7%) (P = .505 between these arms) as compared to those without these mutations (ie, LMR) had TFS at 4 years of 84.7% (95% CI, 71.6% to 92%) on RUX and of 90.6% (95% CI, 78.5% to 96%) on BAT (P = .101 between these arms). The log-rank test was used to compare survival estimates between groups. (E) Forest plot showing multivariable Cox model of TFS. Covariates significant on univariate analysis were included; TP53 mutations, SF mutations, treatment arm, JAK2V617F mutation status, disease duration at trial entry (TE), age, and sex. HMR mutations independently retained negative impact on TFS with a hazard ratio (HR) of 4.21 (P = .006). Treatment arm, JAK2V617F status, disease duration at TE, and age were not significant but, notably, male sex was associated with a poorer TFS (HR, 4.5; P = .006). Driver mutation allele ≥50% was independently associated with a poorer TFS (HR, 4.11; P = .016). Age and disease duration at TE were categorized as continuous variables. Significant P values on forest plot; *P < .05, **P < .01. HMR, high molecular risk (SF and TP53 mutations); JAK2, JAK2V617F; LMR, low molecular risk (without SF or TP53 mutations); SF, splicing factor mutation (SF3B1, ZRSR2, SRSF2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/134/23/10.1182_blood.2019001861/4/m_bloodbld2019001861f2.png?Expires=1769189542&Signature=puCz5AaUwclro6kKE76Pbew2M0rVUACOJyVUuzL~Y5UxP-BKIrqcGeYJ5-xAqnthK~4NR~IAvPNW6XQkhjm0CEWWSk8QqxZ1SX3sCZSM5X4ZapBbVDh~ChVjyu6LVQqBhHHGU8sF2QoWrVQ-xlJWzu5wPSeTem3WM1yTSVoKz-ybUrbCBOm28ziZkV46bqH7nHg7I6bzQnA~OdJbIwJY8rBh-eG1b1js~TltAv~i2hB7oJ-0ZxLWQphN21nTg9da8jSPxQLUKLy92TcTPIRpt~TSpy~TK0PLFog-tnwton2uZef79wU5rjaMSXQY7v6gkkiqaX6KSmvgV41NPxU9XQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal