In this issue of Blood, Yacoub et al1 show that pegylated-rIFN-α2a (PEG) is an effective therapy in patients with myeloproliferative neoplasms (MPN) who are hydroxyurea (HU) intolerant or refractory.
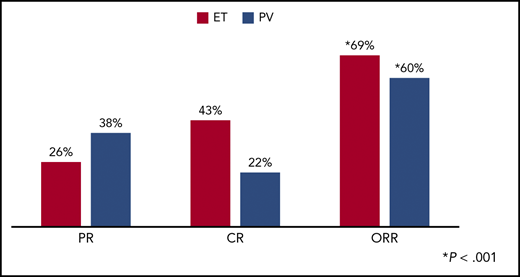
Response rates after 12 months of pegylated interferon alfa-2a in PV and ET. CR, complete response; PR, partial relapse; ORR, overall response rate. See Figure 2 in the article by Yacoub that begins on page 1498.
Response rates after 12 months of pegylated interferon alfa-2a in PV and ET. CR, complete response; PR, partial relapse; ORR, overall response rate. See Figure 2 in the article by Yacoub that begins on page 1498.
In the MPNs polycythemia vera (PV) and essential thrombocythemia (ET), the acquired clone causes excess production of hemopoietic cells. This results in a spectrum of disease characterized by a proliferative bone marrow, increased risk for thromboembolic events and hemorrhage, and in some cases, progression to myelofibrosis and even acute myeloid leukemia. The patients often suffer from a significant symptom burden and detriment of their quality of life. In those who require cytoreductive therapy, the first-line agent is frequently HU. Some patients have an inadequate response to HU so that they do not achieve the targeted reduction in cell counts or they do not tolerate the treatment because of adverse effects. There is evidence that those who are refractory or intolerant to HU have worse outcomes.2 Therefore, there is a major need for other effective treatments.
Interferon α (IFN-α) has shown disease-modifying activity in MPNs and is clearly nonleukemogenic. IFN-α has to be given by injection and has a notable adverse effect profile; however, pegylated forms are given less frequently and are often better tolerated.3,4 Molecular responses have been seen in MPNs.4
In a global, phase 2, investigator-initiated trial of PEG in high-risk patients with PV and ET who were HU resistant or intolerant, Yacoub et al1 showed impressive response rates. In a total study of 115 patients at 12 months, overall response rates in PV were 60%, and 69% in ET (see figure). The criteria for assessment used were the 2009 EuropeanLeukemiaNet criteria,5 which present a high bar to demonstrate response, particularly complete response. In a drug in which adverse effects are a well-recognized issue, the safety profile was acceptable. PEG was well tolerated with discontinuation resulting from adverse events in only 13.9% of patients. The incidence of major thrombotic events at 1 year was 1%, and 5% at 2 years. No major bleeding events occurred during the study. One patient transformed to myelofibrosis during the study, and 1 patient to acute myeloid leukemia within 8 weeks of entering the study. The cumulative incidence of second cancers at 2 years was 4%.
In MPNs, the symptom burden for the patients in an important issue not always appreciated by medical attendants. In this study, statistically significant decreases in MPN-related symptoms were seen. As expected, PEG-related adverse effects occurred, but in those who tolerated treatment, symptom burden from PEG-related adverse effects remained stable through treatment. Patients who achieved a complete response had significantly better MPN-related symptom scores than those with a partial or no response at 12 months.
At the time of the analysis, small but significant reductions in the JAK2V617F allele burden were seen in those in complete response compared with those with no response. A similar pattern was seen with CALR mutations, but did not reach statistical significance, as numbers were small. However, CALR driver mutations were associated with a superior clinical response. In those in which a sample was available for independent histological review, some histopathological remissions were seen.
This trial showed that PEG was an effective therapeutic option, even when using stringent response criteria, in this group of high-risk patients previously treated with HU. In what is a very rare group of patients, this study required an extensive international effort to complete the trial. It represents an important therapeutic advance for this patient group.
It must be recognized that this trial was not randomized. In randomized trials, the difficulty has been treatment options for those who are already HU resistant/intolerant, affecting the results of trials. However, in the light of this trial, the final results of the related phase 3 trial from the Myeloproliferative Neoplasms-Research Consortium (MPN-RC), MPN-RC 112 randomizing between HU and PEG, once treatment is necessary, will be of interest.6 Long-term follow-up of these patients, particularly those who continue long-term on PEG, will also be of great interest. The accompanying small study of patients with MPN with prior splanchnic vein thrombosis who were not necessarily previously treated with HU had an overall response rate with PEG of 70%,7 another important result.
The planned study recruitment was curtailed because of limitations of the PEG drug supply, and the drug supply will be an issue going forward. However, these results are still important, given that another pegylated interferon, ropeginterferon alfa-2b, has been shown to be effective in the treatment of PV8 and is now approved. Ruxolitinib is another therapeutic agent that has been used to treat HU-resistant or HU-intolerant PV and ET. Significant responses have been seen in PV,9 but it was not superior to best available therapy in ET.10 It is unlikely that it will be possible to compare ruxolitinib with PEG directly; nevertheless, the therapeutic armamentarium for patients with MPN has now expanded.
Conflict-of-interest disclosure: The author declares no competing financial interests.