Key Points
Specialized pro-resolving mediators are temporally biosynthesized during venous thrombosis progression.
Resolvin D4 reduces venous thrombosis burden in mice.
Abstract
Deep vein thrombosis (DVT) is a common cardiovascular disease with a major effect on quality of life, and safe and effective therapeutic measures to efficiently reduce existent thrombus burden are scarce. Using a comprehensive targeted liquid chromatography-tandem mass spectrometry-based metabololipidomics approach, we established temporal clusters of endogenously biosynthesized specialized proresolving mediators (SPMs) and proinflammatory and prothrombotic lipid mediators during DVT progression in mice. Administration of resolvin D4 (RvD4), an SPM that was enriched at the natural onset of thrombus resolution, significantly reduced thrombus burden, with significantly less neutrophil infiltration and more proresolving monocytes in the thrombus, as well as an increased number of cells in an early apoptosis state. Moreover, RvD4 promoted the biosynthesis of other D-series resolvins involved in facilitating resolution of inflammation. Neutrophils from RvD4-treated mice were less susceptible to an ionomycin-induced release of neutrophil extracellular traps (NETs), a meshwork of decondensed chromatin lined with histones and neutrophil proteins critical for DVT development. These results suggest that delivery of SPMs, specifically RvD4, modulates the severity of thrombo-inflammatory disease in vivo and improves thrombus resolution.
Introduction
Deep vein thrombosis (DVT) is among the leading causes of permanent disability and mortality worldwide, owing to its major complication: the development of life-threatening pulmonary embolism.1 Postthrombotic syndrome (PTS), characterized histologically by vein wall fibrosis and clinically by symptoms ranging from lower extremity swelling and discomfort to chronic venous stasis ulcer, is another often underappreciated consequence of DVT that significantly reduces quality of life even in the absence of an acute thrombotic event.2 Delayed or incomplete thrombus resolution promotes PTS development.1 Initial acute DVT treatment aimed at thrombus removal includes optimal anticoagulation, catheter-directed thrombolysis, or pharmacomechanical thrombolysis, and these approaches are considered safer than systemic thrombolytic therapies.2 Accordingly, therapeutic strategies aimed at resolving persistent venous thrombi and restoring blood flow to prevent PTS are limited.3 Importantly, currently available standard anticoagulants are beneficial in preventing recurrent thrombosis; however, they are associated with an increased bleeding risk, do not directly lyse established thrombi, and cannot completely prevent PTS development.1
Venous thrombosis (VT) initiation and progression depend on a complex interplay among the coagulation system, erythrocytes, platelets, and endothelial and immune cells. In addition to fibrin fibers and von Willebrand factor strings, neutrophil extracellular traps (NETs) provide an additional important scaffold for binding platelets, red blood cells, and coagulation factors.4 NETs are composed of decondensed chromatin lined with histones and neutrophil enzymes, and are released early during VT development.5,6 Administration of heparin or DNase1 before or immediately after experimental VT initiation protects against DVT.6,7 Although heparin dismantles NETs-derived DNA in vitro,5 and DNase1 efficiently removes protein-free DNA8 and may support NETs clearance by macrophages,9 both heparin and DNase1 are not effective in the removal of proteinaceous NETs components.10 Importantly, presence of NETs may substantially contribute to lysis resistance of thrombi rich in extracellular DNA,11,12 which underlies the necessity for thrombolytic approaches targeting both proteinaceous and DNA-rich components.
VT is a thrombo-inflammatory disease.13 It is now well established that resolution of self-limited inflammation is an active process, involving the endogenous synthesis of specialized pro-resolving mediators (SPMs); namely, the maresins, protectins, resolvins, and lipoxins derived from essential polyunsaturated fatty acids.14 In recent years, their pivotal function in dampening inflammation, tissue remodeling, organ protection, host defense, and microbial clearance has been increasingly recognized.15 SPMs play an important role in the vasculature.16,17 For example, resolvin D1 (RvD1; see supplemental Table 1, available on the Blood Web site, for a list of abbreviations) and RvD2 each decrease vessel wall inflammation, notably by attenuating vascular smooth muscle cell activation,18-20 whereas resolvin E1 (RvE1) reduces adenosine diphosphate-triggered platelet activation and aggregation.21 Another SPM-dependent promising strategy for VT resolution may involve enhancement of macrophage-mediated thrombus removal, as recently indicated by in vitro studies on phagocytosis of clot particles.22,23 Here, we investigate the biosynthesis of SPMs during the progression of DVT in mice, and provide evidence that repetitive delivery of selected SPMs reduces VT burden, a key determinant of PTS development.
Methods
Flow restriction of the inferior vena cava
Experimental procedures in this study were reviewed and approved by the Institutional Animal Care and Use Committee of Boston Children’s Hospital (protocol no. 17-01-3308R) and of Brigham and Women’s Hospital (protocol no. 2016N000145). Eight-week-old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME; Stock 000664; body weight 26 ± 2 g) were anesthetized, and after laparotomy, the inferior vena cava (IVC) was exposed and side branches between the renal and iliac veins were ligated using 7/0 polypropylene suture. The IVC was carefully separated from the abdominal aorta and partially ligated with 7/0 polypropylene suture around a 30-gauge needle that was subsequently removed, which resulted in ∼90% restriction of blood flow.24 For quantification of lipid mediators (LMs), plasma and venous thrombi were harvested at different points after IVC stenosis. In all other experiments, presence of thrombi was verified in the transverse plane, and thrombus length was determined in the longitudinal plane on day 1 postsurgery, using a Vevo 2100 Ultrasound device (FujiFilm VisualSonics). Mice were subsequently separated into 2 groups with similar mean thrombus length. Mice were treated with vehicle (saline solution plus 0.01% vol/vol ethanol) or received a mixture of RvD1, resolvin D4 (RvD4), lipoxin A4 (LXA4), and maresin 1 (MaR1; 500 ng each; Cayman Chemical, Ann Arbor, MI) intravenously (IV) via tail vein injection on day 1, day 3, and day 5. In a different set of experiments, mice were treated with RvD4 (3 µg) IV via tail vein injection on day 1 and day 4. Thrombus size was reanalyzed on day 4 via ultrasound measurements, and plasma and thrombi harvested on day 8. To assess RvD4 loss in whole blood of naive mice after IV injection, RvD4 (3 µg) or vehicle was injected in the tail vein and blood was collected with EDTA (12 mg/tube) at time 0, 5 minutes, 15 minutes, 30 minutes, 1 hour, 24 hours, day 4, and day 8.
LM-SPM metabololipidomics
Plasma (200 µL) and thrombi separated from the vessel wall were snap frozen after harvest. Because of the small net weight of each thrombus (<15 mg), a pool of 3 was used for each experimental condition. Lipid mediators were extracted from the samples and analyzed by liquid chromatography-tandem mass spectrometry, as described.25,26 Experimental details are provided in the supplemental Methods.
Neutrophil isolation and NETosis assay
Neutrophils were isolated from peripheral blood, and NET formation was induced as described.27 A detailed protocol is provided in the supplemental Data.
Histology
Thrombi were excised at day 2, 4, 8, 12, 16, or 21 postsurgery; subsequently fixed in 10% neutral-buffered formalin for 24 hours; and paraffin embedded. Longitudinal sections were cut at 10 µm thickness and hematoxylin and eosin stained.
Flow cytometry
Thrombi were collected on day 8 from thrombus-bearing mice treated with vehicle or RvD4 (3 µg) IV via tail vein injection on day 1 and day 4 after IVC stenosis. Thrombi were washed with phosphate-buffered saline containing Ca2+ (0.9 mM) and Mg2+ (0.5 mM), gently homogenized, and passed through a 70-micron filter. Clot-derived cells were stained for flow cytometric analysis, as described.23 A detailed protocol is provided in the supplemental Methods.
Statistics
Results are mean ± SEM, except for the analyses of the temporal distribution of LM-SPM during DVT progression in mice, where the results represent a pool of 3 thrombi. Statistical analyses were performed with GraphPad Prism software version 6 (GraphPad Software Inc., La Jolla, CA), using 2-tailed paired, unpaired t test, and 1-way ANOVA with Dunnett’s multiple comparisons test. P < .05 was considered statistically significant.
Results
LM profiling reveals a temporal peak in SPM generation during deep vein thrombosis
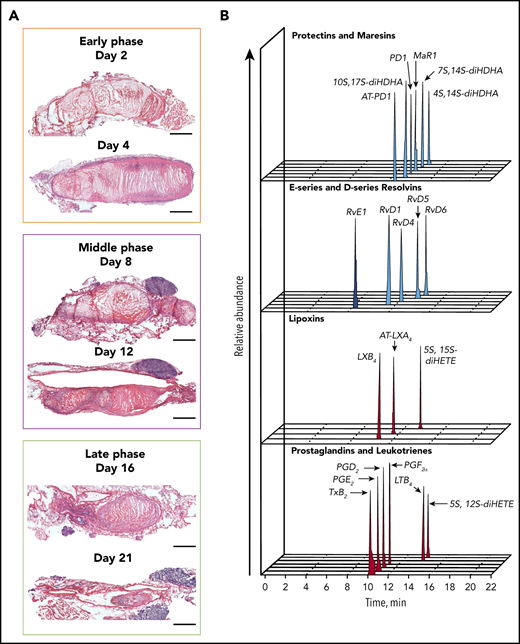
We set out to investigate the LMs profile during the natural course of VT resolution in mice (Figure 1A; supplemental Table 2). During the early (acute) phase of thrombogenesis (up to day 4), the thrombus size peaks and peripheral platelets counts drop significantly (supplemental Figure 1), followed by a subacute phase in the second week after VT induction, in which the thrombus burden gradually decreases (Figure 1A) and its composition changes.28,29 The late (chronic) VT stage is characterized by a significant vein wall remodeling and collagen deposition, whereas the thrombus size is the smallest (Figure 1A).28 Stenosis-induced blood flow restriction triggers local hypoxia, followed by the activation of endothelial cells that release P-selectin and von Willebrand factor, leading to the recruitment of platelets and immune cells. Neutrophils and monocytes contribute to VT initiation by the release of NETs and tissue factor.6,7 Neutrophils constitute two-thirds of the leukocyte population 48 hours after thrombosis induction in mice,6 whereas monocytes and macrophages become the predominant immune cell type during subacute VT stages.28 All these cell types contribute to the biosynthesis of LMs whose levels were determined in thrombi in wild-type (WT) mice at different points between 6 hours and 14 days after IVC stenosis, a stage in resolution at which the thrombus can still be separated from the vessel wall in this model (Figure 1A).
Temporal distribution of LM-SPM in the thrombus during DVT in mice. (A) Naturally occurring VT progression in C57BL/6J WT mice. Hematoxylin and eosin stains of longitudinal mouse venous thrombus sections at days 2, 4, 8, 12, 16, and 21 postinduction. The stenosis site (head of the thrombus) is to the left and the distal end of the thrombus (tail) is to the right on all images. Scale bars, 1 mm. (B) Representative liquid chromatography-tandem mass spectrometry chromatograms of the LMs derived from DHA (blue), from EPA (dark blue), and from AA (red) identified in the thrombus. (C) Tandem mass spectrometry spectra from SPMs identified in the thrombus by characteristic diagnostic ions. (D) Representative principal component analysis score plot (left) and loading plot (right) showing temporal distribution of LMs in the thrombus during DVT. Orange, early (acute) phase (6 hours, day 2, and day 4); purple, middle (subacute) phase (day 6 and day 8); green, late (chronic) phase (day 14) after induction of DVT in WT mice. Data in panels B, C, and D are representative of a pool of 3 thrombi.
Temporal distribution of LM-SPM in the thrombus during DVT in mice. (A) Naturally occurring VT progression in C57BL/6J WT mice. Hematoxylin and eosin stains of longitudinal mouse venous thrombus sections at days 2, 4, 8, 12, 16, and 21 postinduction. The stenosis site (head of the thrombus) is to the left and the distal end of the thrombus (tail) is to the right on all images. Scale bars, 1 mm. (B) Representative liquid chromatography-tandem mass spectrometry chromatograms of the LMs derived from DHA (blue), from EPA (dark blue), and from AA (red) identified in the thrombus. (C) Tandem mass spectrometry spectra from SPMs identified in the thrombus by characteristic diagnostic ions. (D) Representative principal component analysis score plot (left) and loading plot (right) showing temporal distribution of LMs in the thrombus during DVT. Orange, early (acute) phase (6 hours, day 2, and day 4); purple, middle (subacute) phase (day 6 and day 8); green, late (chronic) phase (day 14) after induction of DVT in WT mice. Data in panels B, C, and D are representative of a pool of 3 thrombi.
Using liquid chromatography-tandem mass spectrometry, we identified the presence of specific SPMs in the thrombus (Figure 1B). These proresolving mediators included endogenous protectin D1 (PD1), MaR1, and RvD1, RvD4, RvD5, and RvD6 derived from DHA, RvE1 derived from EPA, and the AA-derived SPMs lipoxin B4 (LXB4) and aspirin-triggered lipoxin A4 (AT-LXA4). Proinflammatory and/or prothrombotic eicosanoids were also identified. These included leukotriene B4 (LTB4), prostaglandins (PG) E2, PGD2 PGF2α, and thromboxane B2 (TxB2, the inactive metabolite of thromboxane A2; Figure 1B; supplemental Table 2). Published criteria were used to identify LMs, which include detection of at least 6 diagnostic ions in the tandem mass spectrometry spectra per mediator and the specific retention time in the multiple reaction monitoring chromatographs (Figure 1B-C).25 Principal component analysis revealed different temporal profiles for these LMs, giving 3 different endogenous clusters for early (acute), middle (subacute), and late (chronic) phase of VT (Figure 1D, left). A first cluster, consisting of RvD4, RvD5, RvD6, MaR1, RvE1, AT-LXA4, AT-protectin D1 (AT-PD1), and LTB4, was associated with the early phase when the thrombus is rich in neutrophils and platelets,29 whereas the production of RvD1, and TxB2 was linked to the middle phase of murine VT. Finally, PD1 and LXB4, together with PGD2, PGE2, and PGF2α, increased during the late stage of murine VT (Figure 1D, right).
We also analyzed the presence of LMs in the plasma of naïve mice (0 hours) at the above noted points in thrombus-bearing mice and in mice that underwent the same surgical procedure, but no thrombus formed in the IVC (supplemental Figure 2; supplemental Table 3). In mice without a thrombus, the variation of some LMs in the early times is mainly a result of local sterile inflammation induced by the surgical procedure, as suggested by results from concomitant analysis of plasma obtained from sham-operated mice harvested during injury-relevant early phases after surgery (supplemental Figure 3). The significant increase in the amount of D-series resolvins RvD5 and RvD6, as well as PD1, in the plasma of thrombus-bearing mice indicated that these molecules may play an important role in thrombus formation. In contrast, the concentration of RvD4, PGD2, and PGF2α, TXB2, and the pathway markers (17-HDHA, 14-HDHA, 18-HEPE, 15-HETE, and 5-HETE) in plasma decreased over time in thrombus-bearing mice (supplemental Figure 2; supplemental Table 3). Compared with the plasma of thrombus-bearing mice, an inverse profile was obtained within the thrombus. The pathway markers were all increased in the thrombus, showing an activation of the SPM pathways (supplemental Figure 2; supplemental Table 2). This reciprocity between the plasma and the thrombus profiles may reflect cellular activation and/or involvement of specific LMs in clot formation within the vessel and ensuing initiation of tissue repair.
Treatment with RvD1, RvD4, LXA4, and MaR1 enhances thrombus resolution
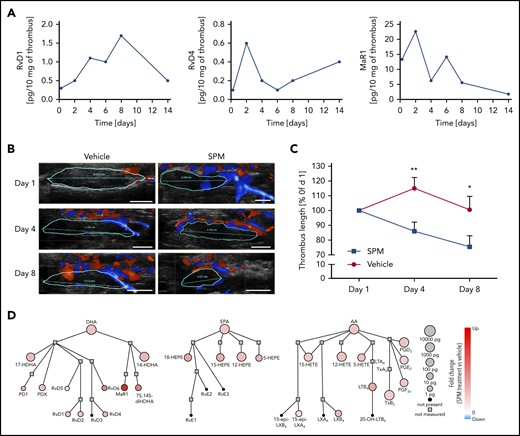
We hypothesized that treatment with a mix containing selected SPMs (RvD1, RvD4, MaR1, LXA4) may arrest thrombus growth and enhance thrombus resolution. RvD4 biosynthesis occurs during coagulation of human blood on removal of adenosine23 and increases the phagocytosis of Escherichia coli by neutrophils and monocytes in human peripheral blood.30 Its amount in the thrombus peaked during the early phases of thrombus development (Figures 1D and 2A; supplemental Table 2), when neutrophils constitute two-thirds of the immune cell population, whereas monocytes represent the remaining one-third.6 In contrast, RvD1 was enriched during the subacute resolution stage (Figures 1D and 2A; supplemental Table 2), when monocytes and macrophages are the predominant immune cell type found in the thrombus.28 RvD1 displays anti-inflammatory/pro-resolving properties in several models of ischemia/reperfusion injury after acute ischemic events,15 inhibits neointimal hyperplasia in a rabbit vein graft model,20 and decreases expression of NETosis markers in an experimental model of abdominal aortic aneurysm.31 MaR1 is biosynthesized predominantly in the early phase of thrombus formation (Figures 1D and 2A; supplemental Table 2), and this is in line with recent results indicating that the interaction between platelets and neutrophils triggers transcellular MaR1 biosynthesis.32 Although LXA4 was not identified in the thrombus at any of the times, we decided to include it in the treatment mix because it is produced by human platelet-neutrophil aggregates, inhibits neutrophil-endothelial interactions, and reduces ischemia/reperfusion-triggered vascular inflammation and the formation of NETs, and its receptor ALX/FPR2 plays an important role in abdominal aortic aneurysm.33-36
Treatment with RvD1, RvD4, LXA4, and MaR1 enhances thrombus resolution in mice. (A) Temporal distribution of RvD1, RvD4, and MaR1 in the thrombus during DVT in mice. Results are expressed in picograms of SPM/10 mg of thrombus and are representative of a pool of 3 thrombi. (B) Representative Doppler ultrasonography images of IVC thrombi obtained from the same mouse at the indicated points after IVC stenosis induction. The red represents arterial flow, the blue is venous flow, and the thrombus is outlined in cyan. Scale bars, 2.5 mm. (C) Relative thrombus length. Thrombus size was monitored by ultrasonography on day 1, day 4, and day 8 postsurgery. n = 7 mice in the vehicle-treated group (●), and n = 9 in the SPM mix-treated group (▪). *P < .05; **P < .01, with 2-tailed unpaired t test for vehicle vs SPM. (D) Network pathway visualization of the LMs in the thrombus after SPM treatment. Node size represents the amount of LM (pg/10 mg of thrombus) in the thrombus after SPM treatment. Node color represents the fold change (compared with vehicle treatment).
Treatment with RvD1, RvD4, LXA4, and MaR1 enhances thrombus resolution in mice. (A) Temporal distribution of RvD1, RvD4, and MaR1 in the thrombus during DVT in mice. Results are expressed in picograms of SPM/10 mg of thrombus and are representative of a pool of 3 thrombi. (B) Representative Doppler ultrasonography images of IVC thrombi obtained from the same mouse at the indicated points after IVC stenosis induction. The red represents arterial flow, the blue is venous flow, and the thrombus is outlined in cyan. Scale bars, 2.5 mm. (C) Relative thrombus length. Thrombus size was monitored by ultrasonography on day 1, day 4, and day 8 postsurgery. n = 7 mice in the vehicle-treated group (●), and n = 9 in the SPM mix-treated group (▪). *P < .05; **P < .01, with 2-tailed unpaired t test for vehicle vs SPM. (D) Network pathway visualization of the LMs in the thrombus after SPM treatment. Node size represents the amount of LM (pg/10 mg of thrombus) in the thrombus after SPM treatment. Node color represents the fold change (compared with vehicle treatment).
WT mice were subjected to the IVC stenosis model of DVT, and thrombus size was determined via Doppler ultrasound on day 1 postsurgery (Figure 2B). Thereafter, mice were separated into 2 groups with similar mean thrombus length at day 1. The first group received vehicle, whereas the second group was treated with a mix of RvD1, RvD4, MaR1, and LXA4 (500 ng each, IV, on days 1, 3 and 5). To quantify the actions of SPM treatment on the rate of early VT resolution, thrombus length was monitored noninvasively on day 4 via Doppler ultrasound and documented on harvest on day 8 when thrombi were excised and processed for further analysis. SPM mix treatment halted thrombus growth and reduced thrombus length by 25% as compared with the vehicle-treated control on day 8 (Figure 2B-C). Network analysis of thrombi on harvest at day 8 indicated that the treatment with a mixture of the endogenous SPMs upregulated DHA, EPA, and AA biosynthesis pathways in the thrombus (Figure 2D).
RvD4 enhances thrombus resolution
On the basis of the results with the combined SPM treatment and earlier findings,30,37 we next focused our attention on the action of RvD4 on VT development. Because RvD4 was specifically enriched during the early organizing phase of thrombosis progression (Figures 1D and 2A), and given its ability to increase neutrophil and monocyte phagocytosis,30 we hypothesized that a supplementation with RvD4 may accelerate thrombus resolution. To test this possibility, we separated mice into 2 groups with a size-matched mean thrombus length 24 hours after thrombosis initiation, treated them with vehicle or RvD4 on day 1 and day 4, and followed thrombus growth via ultrasound sonography (Figure 3A-B). RvD4-treated mice displayed statistically significant reduction in thrombus length on day 8 after IVC stenosis by 17% compared with the vehicle-treated group, demonstrating that repetitive delivery of RvD4 alone accelerates venous thrombus resolution in mice. Repetitive delivery was needed because of the rapid loss of RvD4 in whole blood after IV injection (supplemental Figure 4). Network analysis indicated that RvD4 treatment specifically fueled the biosynthesis of DHA-derived SPM pathways in the thrombus (Figure 3C) and, in contrast to the SPM mix treatment (Figure 2D), did not interfere with the biosynthesis of proinflammatory and prothrombotic mediators.
RvD4 treatment enhances thrombus resolution in mice. (A) Representative Doppler ultrasonography images of IVC thrombi obtained from the same mouse at the indicated time points after IVC stenosis induction. The red represents arterial flow, the blue is venous flow, and the thrombus is outlined in cyan. Scale bars, 2.5 mm. (B) Thrombus size was monitored by ultrasonography on day 1, day 4, and day 8 postsurgery. n = 5 mice in the vehicle-treated group (●), and n = 8 in the RvD4-treated group (▪). *P < .05, with 2-tailed unpaired t test for vehicle vs RvD4. (C) Network pathway visualization of the LMs in the thrombus after RvD4 treatment. Node size represents the amount of LMs (pg/10 mg of thrombus) in the thrombus after RvD4 treatment. Node color represents the fold change (compared with vehicle treatment).
RvD4 treatment enhances thrombus resolution in mice. (A) Representative Doppler ultrasonography images of IVC thrombi obtained from the same mouse at the indicated time points after IVC stenosis induction. The red represents arterial flow, the blue is venous flow, and the thrombus is outlined in cyan. Scale bars, 2.5 mm. (B) Thrombus size was monitored by ultrasonography on day 1, day 4, and day 8 postsurgery. n = 5 mice in the vehicle-treated group (●), and n = 8 in the RvD4-treated group (▪). *P < .05, with 2-tailed unpaired t test for vehicle vs RvD4. (C) Network pathway visualization of the LMs in the thrombus after RvD4 treatment. Node size represents the amount of LMs (pg/10 mg of thrombus) in the thrombus after RvD4 treatment. Node color represents the fold change (compared with vehicle treatment).
Leukocytes are a major component of the thrombus and play an important role in the DVT progression.6 Proresolving actions of RvD4 on human leukocytes, such as the increase of bacterial phagocytosis by human phagocytes (neutrophils, monocytes, and macrophages), have been documented.30,37 Flow cytometry analysis of thrombus revealed that RvD4 significantly decreased the number of CD45+Ly6G+Ly6C−F4/80− neutrophils and CD45+Ly6C+F4/80+ macrophages in the thrombus 8 days after IVC stenosis, whereas it increased the percentage of CD45+Ly6C+F4/80− monocytes (Figure 4A-B). In addition, RvD4 decreased the percentage of live cells by 23% in the thrombus compared with vehicle and increased the percentage of cells in an early apoptosis state (Figure 4C-D). Neutrophils are the predominant immune cell type in mouse venous thrombi at early points6 and contribute to VT progression mainly by releasing NETs that enable platelet and erythrocyte binding.5 We hypothesized that RvD4 may influence the ability of neutrophils to release NETs. Indeed, neutrophils isolated from the blood of mice 24 hours after treatment with RvD4 formed significantly fewer NETs on stimulation with ionomycin compared with neutrophils isolated from mice treated with vehicle (Figure 4E-F). These results were confirmed with mouse neutrophils incubated in vitro with RvD4 before stimulation (supplemental Figure 5). RvD4 did not directly affect platelet aggregation (supplemental Figure 6), suggesting that the main action of RvD4 on venous thrombosis burden is via leukocytes (recruitment, apoptosis, and NETosis).
RvD4 decreases neutrophil and macrophage recruitment in the thrombus and inhibits NETosis. (A, top) Flow cytometry strategy gated on CD45+ leukocytes isolated from mouse thrombi (8 days after IVC stenosis). Monocytes (CD45+Ly6C+F4/80−), macrophages (CD45+Ly6C+F4/80+), and neutrophils (CD45+Ly6GhighLy6C−F4/80−) were identified in the thrombus (8 days after IVC stenosis). (Bottom) Diff-Quik staining of neutrophils, macrophages, and monocytes from mouse thrombus. Scale bar, 20 μm, ×40. (B, top) Percentage of neutrophils, macrophages, and monocytes obtained by flow cytometry in the thrombus 8 days after IVC stenosis of mice treated with vehicle or RvD4. (Bottom) Total neutrophil, macrophage, and monocyte counts in the thrombus. (C) Cell viability in the thrombus 8 days after IVC stenosis was measured using propidium iodide (PI) and annexin V staining. (D) Percentage of live cells (Annexin V−PI−), cells in early apoptosis (Annexin V+PI−), and cells in late apoptosis (Annexin V+PI+) in mouse thrombus (8 days after IVC stenosis) after treatment with vehicle or RvD4. (E-F) Neutrophils were isolated from peripheral blood of vehicle-treated or RvD4-treated mice 24 hours after treatment and kept unstimulated, or were stimulated with ionomycin for 4 hours. (E) Representative fluorescence microscopy images of ionomycin-treated neutrophils illustrating H4Cit+ neutrophils releasing NETs (white arrowheads). H4Cit, green; DNA, blue. (F) Quantification of the percentage of NET-releasing cells. n = 6. Scale bar, 20 µm. *P < .05; **P < .01; ***P < .001 with 2-tailed unpaired t test for comparison of vehicle to RvD4.
RvD4 decreases neutrophil and macrophage recruitment in the thrombus and inhibits NETosis. (A, top) Flow cytometry strategy gated on CD45+ leukocytes isolated from mouse thrombi (8 days after IVC stenosis). Monocytes (CD45+Ly6C+F4/80−), macrophages (CD45+Ly6C+F4/80+), and neutrophils (CD45+Ly6GhighLy6C−F4/80−) were identified in the thrombus (8 days after IVC stenosis). (Bottom) Diff-Quik staining of neutrophils, macrophages, and monocytes from mouse thrombus. Scale bar, 20 μm, ×40. (B, top) Percentage of neutrophils, macrophages, and monocytes obtained by flow cytometry in the thrombus 8 days after IVC stenosis of mice treated with vehicle or RvD4. (Bottom) Total neutrophil, macrophage, and monocyte counts in the thrombus. (C) Cell viability in the thrombus 8 days after IVC stenosis was measured using propidium iodide (PI) and annexin V staining. (D) Percentage of live cells (Annexin V−PI−), cells in early apoptosis (Annexin V+PI−), and cells in late apoptosis (Annexin V+PI+) in mouse thrombus (8 days after IVC stenosis) after treatment with vehicle or RvD4. (E-F) Neutrophils were isolated from peripheral blood of vehicle-treated or RvD4-treated mice 24 hours after treatment and kept unstimulated, or were stimulated with ionomycin for 4 hours. (E) Representative fluorescence microscopy images of ionomycin-treated neutrophils illustrating H4Cit+ neutrophils releasing NETs (white arrowheads). H4Cit, green; DNA, blue. (F) Quantification of the percentage of NET-releasing cells. n = 6. Scale bar, 20 µm. *P < .05; **P < .01; ***P < .001 with 2-tailed unpaired t test for comparison of vehicle to RvD4.
Discussion
This is the first study to document the endogenous biosynthesis of SPMs in thrombi and plasma during the progression of murine DVT, and to demonstrate that SPMs, such as RvD4, significantly enhance VT resolution. Rapid interventions to remove thrombi or their complete resolution are thought to be critical to prevent development of PTS.1 Findings from the present investigations are particularly intriguing, given that venous thrombi are resistant to tissue plasminogen activator-mediated thrombolysis,5 likely because a combination treatment may be necessary that targets both proteinaceous and DNA-rich parts of the thrombus.4
During DVT progression in mice, different LMs were identified in the thrombus and plasma. In general, the LM composition of the thrombus reflects its cellular composition. In the early phase of DVT, during which most of the resolvins and protectins were abundantly produced (Figure 1D), neutrophils and monocytes (cells involved in their biosynthesis) constitute the largest immune cell population in the thrombus.6 Although this early phase is well appreciated to produce thromboxane via platelet activation, a second wave of thromboxane was observed (Figure 1; supplemental Table 2) that may reflect recruitment of monocytes and/or macrophages28 that are also known to produce thromboxane.38-40 Thrombus formation was accompanied by changes in the composition of LMs-SPMs in the plasma (supplemental Figure 2; supplemental Table 3), some of which were decreased in the plasma of thrombus-bearing mice. We hypothesize that the decrease in the plasma and simultaneous increase in the thrombus are related, and that most of the LMs present in the thrombus emanated from entrapped circulating blood cells. Moreover, PD1 (a very potent proresolving LM),41 RvD5, and RvD6 were biosynthesized only in the plasma of thrombus-bearing mice (supplemental Figure 2; supplemental Table 3), probably to counteract DVT-induced inflammation.
Only a few experimental studies have assessed potential novel therapeutic approaches aimed at reducing existent thrombus burden. Kessinger and colleagues demonstrated that statins facilitate resolution of established stasis-induced venous thrombi, which the authors attributed to profibrinolytic, anticoagulant, and antithrombotic effects of the treatment.42 Furthermore, statins given to ApoE−/− mice before IVC ligation were demonstrated to decrease thrombus burden and neutrophil migration into the vessel wall in the early stages of DVT.43 Although it was not addressed in these 2 particular studies, the resolution-promoting function of statins in VT could be further augmented by their anti-inflammatory effects2 or their ability to trigger the biosynthesis of epimeric longer-acting SPMs during neutrophil-endothelial interactions.44 Indeed, it would be worthwhile to assess the effects of combined treatment with statins and LMs or their precursors, as recently reported for patients with coronary artery disease.22,45
Rodent and primate VT models have established another potentially promising strategy to reduce thrombus burden and improve resolution: inhibition of P-selectin,46,47 a protein expressed on the surface of activated platelets and endothelial cells that plays a critical role in thrombosis progression, primarily through its interaction with P-selectin glycoprotein ligand-1 on leukocytes.48 The beneficial effects of P-selectin blockade in thrombus resolution could be a result of multiple factors, including anti-inflammatory and antithrombotic effects and a reduction of P-selectin-dependent NET formation.49,50 Interestingly, LXA4 downregulates the expression of P-selectin on vascular endothelial cells,51,52 and this may further contribute to the proresolving actions of the SPM treatment (Figure 2B-D).
Our results demonstrate that SPM delivery, especially RvD4, halts thrombus growth on treatment initiation on day 1 (Figures 2B-C and 3A-B). The network pathway analysis also revealed that treatment with RvD4 upregulates the DHA-derived SPM pathways in the thrombus, leading to increased concentrations of RvD1, RvD5, RvD6, and PD1 in the thrombus (Figure 3C). This may enhance phagocytosis of clots and apoptotic neutrophils by macrophages, as shown for D-series resolvins and other DHA-derived SPMs in vitro.22,23 Furthermore, multiple SPMs, including RvD1, RvD2, MaR1, LXA4, and PD1, exert distinct anti-inflammatory functions, such as reduction of adhesion molecule expression on the surface of endothelial and immune cells, dampening leukocyte-endothelial cell interactions and the production and release of proinflammatory cytokines.16,17 In the present study, we established that RvD4 decreased the number of CD45+Ly6G+Ly6C−F4/80− neutrophils (Figure 4A-B) and increased the recruitment of CD45+Ly6C+F4/80− monocytes in the thrombus 8 days after IVC stenosis (Figure 4A-B). Thus, RvD4 accelerates the resolution of inflammation by decreasing neutrophil infiltration and increasing recruitment of nonphlogistic monocytes, thus leading to increased efferocytosis and return to homeostasis.14 Moreover, these results demonstrate that RvD4 shares similar recruitment functions on monocytes as LXA4.53 Two mouse monocytes subpopulations can be identified depending on the expression of Ly6C: the classical Ly6C+ inflammatory monocytes and the nonclassical Ly6C− patrolling monocytes.54 Classical monocytes identified in this report are known to be increased in the circulation during systemic or chronic inflammation and recruited to the site of inflammation.55,56 These cells could then differentiate into the Ly6C− monocytes promoting healing and tissue repair.55 Indeed, strategies to enhance the recruitment of monocytes to the thrombus site have been shown to facilitate resolution in VT rodent models.57 Eight days after IVC stenosis, decreased live white blood cells were identified in the thrombus, whereas the level of cells in early apoptosis increased in the RvD4-treated group (Figure 4C-D). Similar to RvE1 and PD1,58,59 RvD4 increased apoptosis, a non-inflammatory cell death.60 Thus, RvD4 affected leukocyte recruitment to the thrombus, but also their viability in the thrombus. Furthermore, our results expand our understanding of the role of RvD4 in neutrophil function by demonstrating that this resolvin significantly reduces NET formation in vitro (Figure 4E-F; supplemental Figure 5). RvD4 appeared to be rapidly lost from whole blood, with approximately 50% of RvD4 lost 30 seconds after injection (supplemental Figure 4). Fifteen minutes after IV injection, RvD4 was still present in the nanogram range in peripheral blood, concentrations known to be active in different in vivo models.20,30,37,61
In general, little is known about the actions of SPMs on NET formation. A recent study demonstrated that treatment of bone marrow-isolated mouse neutrophils with LXA4 also reduces NET release in vitro.35 In the same study, exacerbated NETosis in conjunction with increased mortality after bacterial infection of the lungs was observed in mice deficient for the LXA4 receptor ALX/FPR2.35 Moreover, AT-LXA4 treatment is protective in a mouse model of transfusion-related acute lung injury,62 a disease condition characterized by aberrant NET release.63,64 Likewise, RvD1-treated mice displayed significantly lower levels of citrullinated histone H3, a marker for NETosis, in the aortic tissue of animals subjected to a model of abdominal aortic aneurysm.31 These results and those from our study point toward a novel function of SPMs in modulating NETosis, and may, at least in part, give further mechanistic insight into how treatment with SPMs halts thrombus growth between day 1 and day 4 of mouse VT (Figures 2B-C and 3A-B). Previous studies demonstrated that NET formation becomes increasingly important for thrombus stability between 6 and 48 hours of DVT, with the latter being a point of fulminant NETosis.65 It is therefore likely that an earlier onset of SPM treatment (eg, 6-12 hours postinjury) may have even more pronounced impact in thrombus resolution. An increasing number of studies demonstrate the pathological effect of NETs in many human diseases, including autoimmunity and cancer. Thus, our findings on the effects of SPMs are likely to have a broader effect than DVT.
Of note, mice fed a diet rich in α-linolenic acid (ALA, an ω-3 fatty acid) for 4 weeks showed a similar incidence of stenosis-induced VT occurrence as animals on a control diet.66 However, our study and the ALA study differed in the experimental design: the study by Reiner and colleagues66 assessed whether ALA supplementation for 4 weeks could have a preventive effect on VT induction in mice, whereas the present work specifically focused on resolution-enhancing actions of purified specific SPMs for treatment of existent thrombi. We assume that the tail vein injection route of delivery used here permitted much higher local therapeutic concentrations, which may be essential, as SPMs act locally.17
Despite providing compelling experimental evidence on the role of SPMs in thrombo-inflammatory disease, our study has limitations that need to be considered. Depending on the vessel architecture and branching of the IVC, the murine stenosis-induced DVT model is known to produce thrombus burden of variable size that correlates with different degrees of inflammatory response.28 We therefore analyzed the therapeutic effects of SPMs in groups of thrombus-bearing mice with similar thrombus size as present in the vehicle-treated groups. We focused on the outcome of SPM treatment in the early and middle phase of thrombosis progression, and it would be important to assess their effect throughout the complete resolution of thrombi, and on vessel wall fibrosis and PTS, which is one of the most common and painful chronic consequences of VT.2 Eventually, good experimental models of PTS need to be established and SPMs tested. In addition, site-directed delivery may further enhance efficacy, and the development of specific vascular therapeutics is underway.16,20
In conclusion, SPMs improve the resolution of already-formed murine VT, and these experimental results may have therapeutic potential for thrombo-inflammatory disease. Given their anti-inflammatory and resolution-promoting actions, together with their safety profile,16 and the possibility to be combined with other established treatments,22,45 SPMs such as RvD4 could represent a novel therapeutic approach for thrombo-inflammatory diseases.
In this manuscript, we report no unique sequences or high-throughput datasets. Interested readers that would like to obtain original datasets should write to the corresponding authors (denisa.wagner@childrens.harvard.edu or cserhan@bwh.harvard.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mary Halm Small for expert assistance in manuscript preparation.
This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grants R35HL135765 to D.D.W. and R01GM38765 and P01GM095467 to C.N.S.). D.C. was supported by a research fellowship of the Deutsche Forschungsgemeinschaft (CH 1734/1-1). The funding agencies had no role in gathering, analyzing, or interpreting the data.
Authorship
Contribution: D.C. and C.C.J. designed experiments and wrote the paper; D.C., C.C.J., S.L., E.P.D., L.C., X.d.l.R., and P.C.N. performed and analyzed experiments; D.C. and C.C.J. performed and reviewed the statistics; D.D.W. and C.N.S. conceived and supervised research and critically edited the manuscript; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles N. Serhan, Center for Experimental Therapeutics and Reperfusion Injury, 60 Fenwood Rd BTM 3-016, Boston, MA 02115; e-mail: cserhan@bwh.harvard.edu; Denisa D. Wagner, Program in Cellular and Molecular Medicine, Boston Children’s Hospital, 1 Blackfan Cir, 9th Fl, Boston, MA 02115; e-mail: denisa.wagner@childrens.harvard.edu.
REFERENCES
Author notes
D.C. and C.C.J. contributed equally to this work.
D.D.W. and C.N.S. contributed equally to this work.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal