Abstract
Cancer is the leading cause of death for HIV-infected persons in economically developed countries, even in the era of antiretroviral therapy (ART). Lymphomas remain a leading cause of cancer morbidity and mortality for HIV-infected patients and have increased incidence even in patients optimally treated with ART. Even limited interruptions of ART can lead to CD4 cell nadirs and HIV viremia, and increase the risk of lymphoma. The treatment of lymphoma is now similar for HIV-infected patients and the general population: patients with good HIV control can withstand intensive therapies appropriate to the lymphoma, including autologous and even allogeneic hematopoietic stem cell transplantation. Nonetheless, HIV-related lymphomas have unique aspects, including differences in lymphoma pathogenesis, driven by the presence of HIV, in addition to coinfection with oncogenic viruses. These differences might be exploited in the future to inform therapies. The relative incidences of lymphoma subtypes also differ in the HIV-infected population, and the propensity to advanced stage, aggressive presentation, and extranodal disease is higher. Other unique aspects include the need to avoid potential interactions between ART and chemotherapeutic agents, and the need for HIV-specific supportive care, such as infection prophylaxis. Despite these specific challenges for cancer treatment in the setting of HIV infection, the care of these patients has progressed sufficiently that recent guidelines from the American Society of Clinical Oncology advocate the inclusion of HIV-infected patients alongside HIV− patients in cancer clinical trials when appropriate.
Introduction
Life expectancy in the setting of HIV infection treated with antiretroviral therapy (ART) is now 72 to 75 years, depending on the CD4 count at HIV diagnosis.1 The lifetime cancer risk for these patients is 25% to 40%1-8 ; indeed, cancer is the leading cause of death in economically developed countries,9 and in the United States, non-Hodgkin lymphoma (NHL) is the most common cancer.
In the pre-ART era, NHL risk was 25- to 150-fold higher in HIV-infected persons than in the general population. The risk of NHL remains 11- to 17-fold higher, depending on lymphoma subtype.10 By 75 years of age, the cumulative incidence of NHL in HIV-infected persons is 4.4% compared with 0.01% in HIV− persons.11 This review will focus on the optimization of treatment of HIV-associated NHL (HIV-NHL) and HIV-associated Hodgkin lymphoma (HIV-HL).
Although the lymphoma malignancies reviewed also occur in the HIV− population, their presentation, pathogenesis, and, in some aspects, treatment differ in the HIV-infected patient. Standard curative cancer therapy is the goal and is possible for most of these patients, and, in general, ART can continue during chemotherapy. Table 1 illustrates survival differences for lymphoma subtypes in the pre-ART and current era.
OS of HIV lymphoma subtypes both pre- and post-ART
| . | Pre-ART, % . | Current ART era, % . |
|---|---|---|
| Burkitt lymphoma | 10-4036,57,58,119 | 70-8061,62 |
| DLBCL | 40119 | 70-8036,37 |
| HL | 5587 | 80-9086,87 |
| PBL | 664 | 7568 |
| Primary CNS lymphoma | 2069 | 6073,74 |
| PEL | 3382 | 4082 |
| . | Pre-ART, % . | Current ART era, % . |
|---|---|---|
| Burkitt lymphoma | 10-4036,57,58,119 | 70-8061,62 |
| DLBCL | 40119 | 70-8036,37 |
| HL | 5587 | 80-9086,87 |
| PBL | 664 | 7568 |
| Primary CNS lymphoma | 2069 | 6073,74 |
| PEL | 3382 | 4082 |
CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; PBL, plasmablastic lymphoma; PEL, primary effusion lymphoma.
Lymphoma risk in the HIV-infected population
Pathogenesis of HIV-associated lymphoma
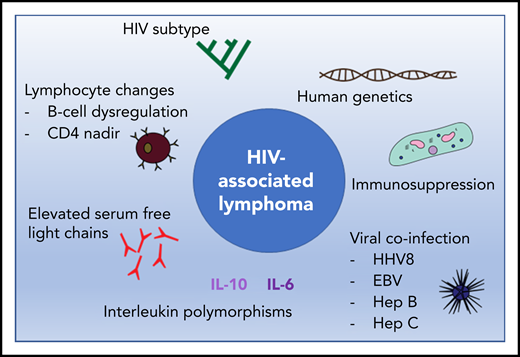
Multiple factors have been shown to influence the pathogenesis of HIV-associated lymphoma (Figure 1). Immunosuppression and coinfection with viruses carrying oncogenic proteins, primarily HHV8 and EBV, contribute to HIV lymphomagenesis, varying by lymphoma subtype.12 For example, CNS lymphoma is virtually always associated with EBV infection; PEL is associated with HHV8 infection and often with EBV infection. AIDS–Burkitt lymphoma is variably associated with EBV infection. The loss of EBV-specific immunity may contribute to lymphomagenesis. For example, 1 study showed the general loss of CD4+ T cells correlated with the loss of EBV-specific CD8+ T cells in subjects progressing to EBV-related NHL.13 Coinfection with hepatitis B or C also appears to increase the risk of NHL14-16 through a mechanism that includes chronic immune stimulation, although the precise pathway remains to be elucidated.
The pathogenesis of HIV-associated lymphoma is complex and influenced by host-mediated and viral factors. EBV, Epstein-Barr virus; Hep B, hepatitis B; Hep C, hepatitis C; HHV8, human herpes virus 8; IL-6, interleukin 6; IL-10, interleukin 10.
The pathogenesis of HIV-associated lymphoma is complex and influenced by host-mediated and viral factors. EBV, Epstein-Barr virus; Hep B, hepatitis B; Hep C, hepatitis C; HHV8, human herpes virus 8; IL-6, interleukin 6; IL-10, interleukin 10.
Several changes in cell biology are known to precede lymphoma but are not currently used for diagnosis. B-cell dysregulation secondary to HIV infection contributes to lymphomagenesis. Elevated levels of serum free light chains (SFLCs) increase by eightfold the risk of NHL,17 whereas the ratio of SFLCs, total immunoglobulin, and immunofixation are not predictive of NHL. Unfortunately, SFLC level is insufficiently predictive for routine screening of asymptomatic HIV patients.18 Interleukins including IL-10 and IL-6 are also elevated prior to a diagnosis of HIV lymphoma,19 and although single-nucleotide polymorphisms in IL-10 may predispose to lymphoma,20 it is not currently standard practice to prospectively screen for elevated IL-10.
In cohort studies, HIV viremia and depth of CD4 nadir increase lymphoma risk.21 Even relatively short planned ART interruptions in a randomized drug conservation trial increased cancer risk by sixfold and lymphoma risk by 3.7-fold.22 Indeed, HIV may directly drive lymphogenesis.23 The HIV-1 matrix protein p17 persists in germinal centers after HIV-1 drug suppression; its variants (vp17s) activate Akt signaling, promoting transformed B-cell growth, and may also upregulate the EBV oncoprotein latent membrane protein 1 (LMP-1) in EBV-infected B lymphocytes, leading to lymphoma.
All of these disease-associated factors contribute to genetic differences between HIV and non-HIV lymphoma, such as interstitial deletion of fragile site-associated genes, and other changes identified in genome-wide DNA-profiling studies,24 but no therapeutic strategies have emerged to capitalize on them. Inherited genetic factors play a further role in the development of NHL, although genetic screening is not part of current clinical practice. For example, the chemokine receptor CCR5 is a major coreceptor for the entry of M-tropic variants of HIV-1, and homozygosity of a 32-bp deletion (CCR5-D32, present in 1% of a white population) confers resistance to HIV-1.25 In addition, HIV-infected patients heterozygous for the CCR5-D32 deletion are less likely to develop lymphoma. Conversely, stromal-cell–derived factor-1 mutation increases lymphoma risk.26
Subtypes of HIV-associated NHL
The World Health Organization Classification System recognizes HIV-NHL subtypes.27 More than 95% are of B-cell origin, including DLBCL, Burkitt lymphoma, and PBL. Rare types include T-cell lymphomas and PELs, which lack B-, T-, and hematopoietic cell markers. Primary CNS lymphoma, a rare B-cell subtype more commonly seen in the early years of the AIDS epidemic, is rarely diagnosed when ART is used consistently.
Dominant clinical features across all subtypes of HIV-NHL include stage III or IV disease, rapidly growing masses, and B symptoms (ie, fever, night sweats, and unexplained weight loss).28 Extranodal involvement includes the bone marrow in 25% to 40%, the gastrointestinal tract in 26%, and the CNS in 17% to 32% of cases. Leptomeningeal disease may be detected by cerebrospinal fluid (CSF) flow despite negative cytology at a higher frequency than in the immunocompetent population, where it is rare. In 1 study of 4-parameter flow (CD3, CD19, κ, and λ) in 51 newly diagnosed DLBCL patients, 11 (22%) had leptomeningeal disease detected by flow cytometry alone and only 1 was detected by cytology.29 Detection may be higher now with ultrasensitive modern multiparameter flow cytometry.
Lymphoma treatment strategies in HIV-infected patients
DLBCL
DLBCL is the most common aggressive lymphoma irrespective of HIV status. However, HIV-DLBCL is more commonly associated with the MYC and BCL6 translocations and with proliferation indices >80%.30,31 Prior to ART, HIV-DLBCL patients experienced significant toxicity and shorter remissions on chemotherapy, related to their advanced HIV infection.32 Thus, reduced-intensity chemotherapy became the standard of care, but this approach is now obsolete with current ART.
Currently, except when treating rare stage I, low-risk patients, who often receive rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) combination chemotherapy and radiation, the backbone of ongoing clinical trials at the AIDS Malignancy Consortium (AMC) is combination chemotherapy rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin–cyclophosphamide dose-adjusted to CD4 count (R-EPOCH). Various combination chemotherapies studied in HIV-DLBCL include doxorubicin, cyclophosphamide, vindesine, bleomycin, methylprednisolone, and methotrexate (ACVB) and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), studied in a risk-adapted trial,33 as well as the infusional regimen cyclophosphamide, doxorubicin, and etoposide (CDE).34,35 Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH), an infusional regimen given over 5 days, emerged as the predominant approach after a 2003 US National Cancer Institute (NCI) study showed a 79% complete remission (CR) rate.36
Building on EPOCH, AMC trial AMC-034 favored the addition of concurrent over sequential rituximab, the anti-CD20 antibody37 ; the study found a 12% rate of febrile neutropenia, a 28% rate of any grade 3 or 4 infection, and 2-year rates of progression-free survival (PFS) and overall survival (OS) in the range of 70%. A pooled analysis of 1546 patients in 19 clinical trials strongly favored R-EPOCH over R-CHOP38 for event-free survival (EFS) (hazard ratio [HR], 0.40; 95% confidence interval, 0.23-0.69; P < .001) and OS (HR for death, 0.38; 95% confidence interval, 0.21-0.69; P < .01). R-EPOCH may overcome the high proliferation index (>80%) in HIV-related lymphoma. In fact, proliferation marker Ki-67 >90% was associated with better survival in a comparison of R-EPOCH and R-CHOP in 2 AMC phase 2 studies (AMC-010 and AMC-034),39 although these results may be confounded by changes in ART over time (neither study mandated ART) and by differences in baseline CD4 counts. In contrast, a recent French retrospective study found R-CHOP and R-EPOCH were equivalent in HIV-DLBCL, although it included only 52 patients.40 Moreover, in the general population, R-EPOCH and R-CHOP were equivalent in a 2016 randomized trial, although subset analysis is pending.41
CNS prophylaxis in DLBCL
CNS prophylaxis is used in HIV-DLBCL and has the same indications as in the general population. Studies have not been done to refine the indications or determine optimal regimens specifically for HIV-DLBCL. Notably, intrathecal (IT) or systemic strategies are variously used in HIV− patients, but most HIV-DLBCL patients receive CNS prophylaxis because of the prevalence of extranodal disease. Flow cytometry should be used at least on the initial CSF sample to rule out cytologically negative occult disease. Patients with occult disease treated with prophylactic schedules alone did not do well,29 suggesting that treatment approaches for active CNS involvement may be warranted.
Anti-CD20 monoclonal antibody therapy in B-cell lymphoma
Rituximab is a ubiquitous component of B-cell NHL therapy in the general population, and although initial phase 2 studies suggested safety issues in HIV, these have been discounted and this drug is now widely used and recommended.42-44 A 2005 phase 3 study failed to demonstrate any added benefit of rituximab added to CHOP and suggested increased toxicity for patients with CD4 counts <50 cells per microliter.45 However, a pooled analysis of AMC studies suggested treatment-related mortality (TRM) was unrelated to the rituximab, finding 37% TRM in all patients with CD4 count <50 cells per microliter compared with only 6% TRM in the remaining patients (P < .01).46 Noncomparison trials have also contributed to the general consensus to use rituximab while monitoring for infection, using prophylactic hematopoietic growth factor therapy in all patients and antibiotic prophylaxis in those at highest risk.44,47-49
The role of immune status in DLBCL
HIV-related immune status influences not only the incidence of DLBCL but also its outcome. An HIV-specific lymphoma score consists of 3 components: age-adjusted International Prognostic Index score; number of involved extranodal sites; and an HIV score that incorporates baseline CD4 count, HIV viral load, and prior history of AIDS.50 Additional tumor markers may also be prognostic: Ki-67, CD44, CD20, EBV, cMYC proto-oncogene protein, S-phase kinase-associated protein 2 (SKP2), B-cell lymphoma 6 [BCL6] protein, tumor protein p53, and immunoglobulin M. Patients who are naive to ART at the time of lymphoma diagnosis may do well, however, if immune reconstitution on ART occurs during or subsequent to chemotherapy (see discussion of concurrent ART in “Infection prophylaxis” below).51,52 In contrast, in patients with advanced HIV infection, marked by profoundly low CD4 counts and multidrug-resistant HIV, the CD4 count may never recover after chemotherapy.
Cell of origin in DLBCL
Cell of origin is prognostic in DLBCL in the general population.53 HIV− DLBCL can be classified according to its cell of origin by gene-expression profiling as germinal center B-cell–like (GCB) subtype or activated B-cell–like subtype, and approximated by immunohistochemistry as GCB and non-GCB subtypes where the Hans model predominates.54 Applying this approach to HIV-DLBCL has shown conflicting results. For example, 5-year PFS with only 3 cycles of short-course etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin with dose-dense rituximab (RR-EPOCH) was reported as 95% in GCB-subtype compared with 44% in non-GCB–subtype HIV-DLBCL.55 However, this treatment approach for advanced-stage HIV-DLBCL has not been investigated in another trial. In contrast, a retrospective analysis of several AMC trials using 6 cycles of R-EPOCH showed the same survival in GCB and non-GCB subtypes.39 Analyzed by protein expression, AIDS-related DLBCL shared features of both germinal center and nongerminal subtypes, suggesting a pathophysiology distinctive from HIV− DLBCL.56 The reason for this difference is unclear, although it is possible that viral coinfection influences pathogenesis, as noted in "Pathogenesis of HIV-associated lymphoma."
Burkitt lymphoma
Burkitt lymphoma is highly overrepresented in patients living with HIV. The mutations found in AIDS-related Burkitt lymphoma include those found in sporadic Burkitt lymphoma: activating mutations in the cMYC proto-oncogene, frequent inactivation of p53, and point mutations in BCL6. EBV is more prevalent in HIV–Burkitt lymphoma, present in 25% to 40% of cases. As in DLBCL, intensive therapies standard in the general population were avoided in HIV-related cases of Burkitt lymphoma before the ART era because of fears of TRM. Challenging the prevailing dogma, a retrospective study of cyclophosphamide, vincristine, doxorubicin, and methotrexate/ifosfamide, etoposide, and cytarabine (CODOX-M/IVAC) administered with ART showed a 2-year EFS of 80%.57 A prospective study of 13 patients treated with cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) plus methotrexate and high-dose cytarabine with ART showed a 92% complete response rate but a 2-year OS rate of only 38%,58 results similar to those reported by others.59,60 The AMC-048 trial, the largest prospective study dedicated to HIV–Burkitt lymphoma, added rituximab and modified the dosing and schedules of CODOX-M/IVAC to reduce toxicity.61 The 2-year OS rate was 69% among 34 patients in the study and only 1 treatment-related death was reported. Modifications of the CODOX-M/IVAC regimen resulted in a grade 3 to 4 toxicity rate of 79%, lower than the 100% rate of the parent regimen, and without grade 3 to 4 mucositis. Despite a 68% protocol completion rate, the 1-year survival rate compares favorably with studies that excluded HIV+ patients. Notably, no patients with parenchymal CNS disease were enrolled, though they were eligible.
The US NCI reported favorable results with dose-adjusted R-EPOCH for both HIV-infected and HIV− patients with Burkitt lymphoma, as well as less toxicity compared with prior intensive regimens.62 A confirmatory multicenter trial, reported in abstract form, included 28 HIV-infected patients (25%), supporting the use of this regimen in patients without parenchymal CNS disease, a study exclusion.63 HIV status did not affect the projected 2-year EFS rate of 85%. The cytotoxic agents in R-EPOCH do not cross the blood-brain barrier, and this regimen cannot be used in the setting of evident brain metastases; regimens that incorporate CNS treatment are preferable in this situation. Leptomeningeal disease was allowed and present in 25% of patients; leptomeningeal disease, peripheral blood, and bone marrow involvement at diagnosis predicted treatment failure. When all 3 factors were present, the 1-year EFS was 65%, compared with 98% when no factor was present. A direct comparison of this trial to R-CODOX-M/IVAC is not possible. However, the primarily outpatient R-EPOCH regimen is associated with less hematologic toxicity by design, and, in the absence of a randomized trial, it is a reasonable strategy for patients without parenchymal CNS involvement. The Stichting Hemato-Oncologie voor Volwassenen Nederland (HOVON) group is conducting a randomized trial in Europe to compare R-CODOX-M/IVAC with R-EPOCH.
CNS prophylaxis via IT treatment is mandatory in Burkitt lymphoma irrespective of HIV status unless the patient has low-risk disease.63 The multicenter R-EPOCH trial omitted IT prophylaxis in low-risk patients, defined as having a single site of disease <10 cm in diameter and normal lactate dehydrogenase, or complete resection and a negative prechemotherapy CSF by flow cytometry and cytology. These patients had no CNS relapses.
Plasmablastic lymphoma
Plasmablastic lymphoma was originally described in the pre-ART era as nearly exclusively associated with HIV and almost entirely incurable.64 This very rare CD20− variant of HIV-DLBCL has myeloma markers (including CD38 and CD138) and a proliferation index typically >90%. Although many patients present with a stage I jaw mass, others have disseminated disease including numerous bone lesions. Retrospective studies suggest curability in the ART era in both HIV-infected and HIV− patients,65,66 although a dedicated prospective study has not been conducted because of the rarity of the disease. By default, most experts use EPOCH to treat PBL, and there are some anecdotal reports adding bortezomib to EPOCH because of its activity in myeloma.67 A recent prospective AMC study included 15 PBL patients, 10 with CD4 counts <200 cells per microliter; the rate of both complete response and 1-year OS using EPOCH alone was 67%68 (Juan Carlos Ramos, manuscript submitted September 2019). The disease must be differentiated from myeloma, which has a different therapeutic approach.
Primary CNS lymphoma
Primary CNS NHL typically affects patients with CD4 counts <50 cells per microliter and is rarely seen in patients receiving ART. Most cases are DLBCLs, EBV-associated, and multifocal.69 Indeed, EBV in the CSF can assist in making the diagnosis when biopsy is infeasible, especially when combined with 201Tl single-photon-emission computed tomography. Patients present with symptoms of confusion, memory loss, lethargy, and other focal neurologic findings.70 In the pre-ART era, palliative treatment of steroids and whole-brain radiation (3000-5400 Gy) led to response rates of 75% and a median OS of 2 to 4 months regardless of CD4 count.71 A pilot study using only zidovudine (1.5 g twice daily), ganciclovir (5 mg/kg twice daily), and IL-2 (2 000 000 U twice daily) had 2 of 5 patients remaining in CR at 28 months and 52 months, but closed early because of low patient accrual.72 Induction regimens are generally based on high-dose methotrexate, and retrospective studies of AIDS-related primary CNS lymphoma include 14 ART-era patients whose 5-year OS was 60%,73 and 51 ART-era patients whose 5-year OS was 48%.74 Several novel approaches are available for HIV− primary CNS NHL, but there are no data on their effectiveness in the setting of HIV. For example, although it excluded HIV-infected patients, a recent study of sequential multiagent induction chemotherapy and upfront autologous stem cell transplant demonstrated an 81% 2-year OS in primary CNS lymphoma, suggesting that this approach could be attempted in the appropriate HIV-patient setting.75
PEL
PEL comprises ∼1% to 5% of AIDS-related lymphoma cases. Typically, diagnosis of a malignant effusion without nodal disease is made by pericardiocentesis, thoracentesis, or paracentesis using cytology and immunohistochemistry. The PEL cells are pleomorphic and lack expression of B-cell–associated genes, including surface immunoglobulin, although they harbor immunoglobulin gene rearrangements. The gene-expression profile of PEL cells suggests plasmablastic derivation.76 The malignant clone is almost always found to be HHV8-infected, with latent gene products contributing to pathogenesis.77-81 Concurrent EBV infection is frequent. Furthermore, PEL may extend into tissues underlying the serous cavities, including the omentum, lymph nodes, mediastinum, and lung. In a retrospective analysis of 11 PEL patients treated with doxorubicin, vincristine, and prednisone, the CR rate was 42% but median survival was only 6 months.82 As with other lymphomas, the outcome may be better in the ART era,83 although there are no prospective studies of PEL. A report of antiviral therapy alone leading to sustained remission has been documented84 but this treatment is not the standard of care.
HL
HIV infection markedly increases the risk of HL, although it is not an AIDS-defining diagnosis. In fact, ART and immune reconstitution increase the incidence of HL, whereas ART decreases the incidence of NHL.85 HIV-HL is associated with B symptoms, mixed cellularity subtype more commonly than nodular sclerosis subtype, and extranodal disease.86 Unlike in HIV− HL, in HIV-HL, the noncontiguous spread of disease is common and there is far less mediastinal involvement.87 Bone marrow involvement in 40% to 50% of patients may be the first indication of HIV-HL in patients with B symptoms or cytopenias. AIDS-HL, like AIDS-NHL, is characterized by a high frequency of EBV infection when compared with HIV− HL. The pathologic role of EBV in HL is evidenced by the Reed-Sternberg cell expression of EBV-transforming proteins.
Before the ART era, the response to standard chemotherapy doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and OS rate (median, 1.5 years) were lower in HIV-infected patients with HL. Similar to HIV-NHL, in antiviral-naive HIV-HL patients, the response to ART correlates with outcome.88 In the ART era, outcomes with HIV-HL are comparable to those in the general population: a French study showed 2-year OS and PFS of 94% and 89%, respectively,86 and a US intergroup study (S0816) showed similar results in a small cohort.89 A German study of 108 patients using a stratified treatment approach, including bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (BEACOPP) for the advanced-stage patients, showed CR rates for early-stage favorable HL, early-stage unfavorable HL, and advanced-stage HL of 96%, 100%, and 86%, respectively. The study reported 2-year PFS and OS rates of 92% and 91%, respectively, after a median follow-up of 26 months.90 However, BEACOPP is rarely used in frontline therapy in the United States because of its toxicity. The US Food and Drug Administration (FDA) recently approved the antibody-drug conjugate brentuximab vedotin (BV) in combination with doxorubicin, vinblastine, and dacarbazine (AVD) for stage III and stage IV HL in the general population, after a phase 3 study91 showed 2-year modified PFS rates of 82.1% and 77.2% (P = .04) for the BV plus AVD and ABVD-only arms, respectively. The BV combination decreased the risk of disease progression and death (HR, 0.770; P = .035), whereas OS was the same. The AMC is accruing untreated patients with stage II to stage IV HIV-HL to a study of AVD-BV, after a phase 1 study demonstrated no dose-limiting toxicities in 6 patients.92 HIV-specific concerns for this combination compared with ABVD include the increased risk of neuropathy.
MCD
Though not a clonal disorder, multicentric Castleman disease (MCD) has a similar presentation to lymphoma, including multifocal adenopathy, effusions, and lymphoma-type systemic symptoms.93 The HHV8 infection of plasmablasts, liberating viral IL-6 into the bloodstream, is central to the pathogenesis of HIV-related MCD and distinguishes it from idiopathic MCD, in which endogenous IL-6 is elevated. Moreover, the plasmablasts retain CD20 expression in HIV-related disease, enabling therapeutic targeting with rituximab. Intermittent therapy for relapsing disease is a reasonable strategy for prolonged disease management.94 MCD can precede HHV8-related DLBCL, and repeat biopsy is indicated for relapsed or refractory disease.
Considerations for specific therapies in the comorbid setting
Transplantation and chimeric antigen receptor T cells for HL and NHL
Myeloablative autologous transplants have been performed in both NHL and HL patients. The City of Hope reported the first prospective study, in which 9 of 12 NHL patients beyond first CR were alive at 18.5 months after transplant.95 Other pilot studies included 8 patients96 and 6 patients.97 The European Cooperative Study Group on AIDS and Tumors (GICAT) reported the only study enrolling patients before second-line therapy, in which 27 of 50 eligible lymphoma patients received autologous transplant and median OS for the entire group was 33 months.98 In addition, 3 retrospective case-control studies showed that outcomes after autotransplant were equivalent irrespective of HIV status.99-101 These trials laid the groundwork for the AMC and Blood and Marrow Transplant Clinical Trials Network (BMT CTN) multicenter study, which demonstrated a 1-year TRM of 5.2% and a 2-year OS of 87% for HIV-infected patients after autotranspalnt.102 Outcomes did not differ between the 43 trial patients and 153 HIV− controls from the Center for International Blood and Marrow Transplant Research database, leading to the conclusion that HIV patients should receive autologous transplantation when appropriate to the management of their lymphoma.
Nonmyeloablative allogeneic transplantation has been used in select patients with refractory disease and produced short-term remissions (12 months) with little toxicity, including few opportunistic infections.103 A retrospective study of 23 patients suggested outcomes would improve in the ART era.104 The AMC and BMT CTN recently reported a pilot study of allogeneic transplant in a wide variety of hematologic malignancies.105 Conditioning was myeloablative (n = 8) or reduced-intensity (n = 9), investigators were encouraged to continue ART throughout the transplant process whenever possible, and, at 100 days, TRM was 0. Complete chimerism was achieved by 8 patients by 100 days, and by 1 additional patient by 6 months. HIV was detected by a viral outgrowth assay for very low viremia in the 2 patients with mixed chimerism but was not detected in either of the 2 patients who achieved 100% donor status. Median follow-up of survivors was 24 months (range, 7-27 months). The cumulative incidence of acute graft-versus-host disease grade II-IV was 41%, 1-year OS was 59%, and the causes of death were relapsed/progressive disease (n = 5), acute GVHD (n = 1), adult respiratory distress syndrome (n = 1), and liver failure (n = 1). Infections were reported in 11 patients (n = 3, grade 2; n = 8, grade 3).
One patient with acute myelogenous leukemia, also known as the Berlin patient, received a transplant from a donor with a homozygous deletion in CCR5 and has probably been cured of HIV.106 As noted in "Pathogenesis of HIV-associated lymphoma," CCR5 is a major coreceptor for the entry of M-tropic variants of HIV-1. Thus, a donor homozygous for a 32-bp deletion (CCR5-D32) could theoretically repopulate the bone marrow with cells resistant to HIV.106 To date, large-scale trials exploring HIV-resistant donors have not been feasible.
Notably, autologous chimeric antigen receptor T-cell strategies against CD19 have not been explored in HIV-infected patients, who were excluded from these clinical trials. Future studies could explore what level of immunity is necessary to support this approach.
Infection prophylaxis
All HIV patients should receive hematopoietic growth factor support to prevent neutropenia and secondary infection. The frequency and duration of this support depends on the intensity of the regimen and the immune status of the patient. Neutropenia should be avoided if possible in patients with a CD4 count <50 cells per microliter because it increases their risk of septic shock.45,46
Other infection prophylaxis is given on the basis of known risks associated with varying degrees of immunosuppression.107 All HIV patients treated with chemotherapy should receive Pneumocystis prophylaxis regardless of CD4 count. Systemic prophylaxis rather than inhaled pentamidine is preferable. Prophylaxes for herpes simplex and varicella are needed when patients receive intensive chemotherapy regimens. All patients with a CD4 count <100 cells per microliter should be considered for quinolone prophylaxis during nadir, especially if rituximab is used, because of excess sepsis-related deaths in this population. Fluconazole may be given for thrush but should be avoided 1 day before and after chemotherapy because it interferes with clearance of drugs metabolized through cytochrome P450 3A4 (CYP3A4).108
HIV-infected patients have an increased prevalence of viral hepatitis B. Screening for hepatitis B is particularly important prior to treatment with rituximab-containing regimens. Rituximab can lead to reactivation of latent hepatitis B virus or exacerbation of low-level infection, both leading to fulminant hepatic failure. Patients who screen positive for hepatitis B must be on antiviral therapy for hepatitis B, often accomplished with drugs that overlap with anti-HIV therapy. Care should be taken to avoid hepatitis B prophylaxis without highly active antiretroviral therapy to avoid outgrowth of resistant HIV strains. Typically, anti–hepatitis B therapy is continued for at least a year after rituximab exposure.109
Combining chemotherapy and antiviral therapy
Most oncologists continue ART during chemotherapy, whereas some temporarily discontinue it, citing concerns regarding drug-drug interactions and increased toxicities. Indeed, in support of concurrent chemotherapy and ART, 6 randomized studies of opportunistic infections showed that immune recovery within the first month of ART decreases infection mortality.110 Thus, discontinuing or postponing ART initiation during chemotherapy could be detrimental.
Concurrent ART is associated with more rapid immune reconstitution after chemotherapy completion: T-helper cell and natural killer cell counts typically recover to pretreatment levels within 1 month of finishing chemotherapy.111 In AMC-034, R-EPOCH ART was used at physicians’ discretion.112 A retrospective analysis showed that concurrent ART therapy did not affect infection rates or lymphoma-specific outcomes, but immune recovery was accelerated in the concurrent ART cohort, particularly postchemotherapy.
With new ART medications available, it is now possible to avoid specific ART components to minimize drug-drug interaction (see known interactions and considerations in Table 2). Potentiation of neurotoxicity and myelosuppression deserves careful attention. National Comprehensive Cancer Network (NCCN) guidelines provide additional details (www.nccn.org). Practitioners should consult with pharmacologists and other resources about new drugs. Generally, regimens based on integrase inhibitors are combinable with chemotherapy. Given the availability of ART and evidence of improved immune recovery, it is extremely unlikely that a randomized trial with or without ART will ever be conducted.
Examples of chemotherapy and antiviral interactions
| Drug . | Effect . | Recommendation . |
|---|---|---|
| Cobicistat | Strong CYP3A4 inhibitor that will increase chemotherapy toxicity | Avoid with chemotherapy such as doxorubicin or vinca alkaloids |
| Fluconazole | CYP3A4 inhibitor that will increase chemotherapy toxicity | Avoid before and during doxorubicin or vinca alkaloids therapy |
| Ritonavir | Strong CYP3A4 inhibitor that will increase chemotherapy toxicity | Avoid with chemotherapy such as doxorubicin or vinca alkaloids |
| Zidovudine | Myelosuppression | Contraindicated with chemotherapy |
| Drug . | Effect . | Recommendation . |
|---|---|---|
| Cobicistat | Strong CYP3A4 inhibitor that will increase chemotherapy toxicity | Avoid with chemotherapy such as doxorubicin or vinca alkaloids |
| Fluconazole | CYP3A4 inhibitor that will increase chemotherapy toxicity | Avoid before and during doxorubicin or vinca alkaloids therapy |
| Ritonavir | Strong CYP3A4 inhibitor that will increase chemotherapy toxicity | Avoid with chemotherapy such as doxorubicin or vinca alkaloids |
| Zidovudine | Myelosuppression | Contraindicated with chemotherapy |
Gene therapy
Patients already undergoing autologous stem cell transplant for relapsed and refractory lymphoma have been studied in an early trial of gene therapy to engineer reinfused stem cells resistant to HIV.113 Four patients received a lentivirus vector with 3 RNA-based anti-HIV moieties (tat/rev short hairpin RNA, transactivating response decoy, and CCR5 ribozyme). Low levels of transcripts were detectable at 24 months. This pilot study was not designed to eradicate HIV, only to explore the feasibility of gene therapy. Gene therapy is an active area of research with newer vectors and anticipated higher rates of transduction (AMC-097 clinicaltrials.gov identifier: NCT02797470). If AMC-097 is successful, future trials could explore similarly transduced autologous hematopoietic stem cells in HIV patients without malignancy in an effort to cure HIV infection.
Eligibility in general population clinical trials
The American Society of Clinical Oncology has convened expert panels to issue policy statements on clinical trial eligibility in underserved populations including HIV-infected patients.114 The main recommendations were that HIV patients who are healthy and at low risk for AIDS-related outcomes should be included in general population patient studies. HIV-related eligibility criteria should be straightforward and focus on current and past CD4 and T-cell counts, history (if any) of AIDS-defining conditions, and status of HIV treatment. HIV-infected patients should be treated using the same standards as other patients with comorbidities, and ART should be considered a concomitant medication. Notably, investigators should take into account the immune status of general population patients on the trial and not discriminate based on CD4 count alone. Finally, ART chemotherapy interactions should be evaluated on a trial-by-trial basis.
HIV lymphoma in limited-resource settings
In sub-Saharan Africa, the AIDS epidemic has led to a marked increase in the burden of HIV/AIDS-related malignancies. Treatment of lymphoma in these settings is constrained by resources that limit both the availability of IV chemotherapy and the supportive care of complications. For example, in Burkitt lymphoma, IV systemic therapy is associated with mortality rates between 20% and 66%.115-117 With the goal of limiting neutropenia and its complications, a study in Uganda and Kenya used modified oral chemotherapy to treat HIV-DLBCL.118 Only 4 febrile neutropenia episodes and 3 treatment-related deaths (6% mortality rate) occurred. Although the median survival was only 12 months, 33% of patients survived 5 years. Only 18 of 49 patients (37%) had access to ART, and those patients had nearly a threefold increase in survival (P = .035). The AMC has an ongoing randomized study in sub-Saharan Africa comparing standard CHOP with an oral chemotherapy regimen (AMC-068).
Conclusion
Life expectancy in HIV-infected persons on ART is approaching that of the general population. Consequently, both AIDS-defining and non–AIDS-defining cancers have become the largest source of mortality. Interruptions in HIV treatment should be avoided as they lead to increased cancer risk. Although NHL incidence has declined with ART, and the subtypes of NHL have shifted, NHL and HL remain disproportionally prevalent among HIV-infected patients. Notably, CNS lymphoma is now very rare in patients on ART. Intensive curative cancer therapies are now the standard of care, except in patients with advanced AIDS. These therapies include transplant strategies in the setting of relapsed and refractory lymphoma. Future directions include exploiting biologic differences to therapeutic advantage: for example, targeting EBV and/or HHV8 coinfection; engineering hematopoietic stem cells with anti-HIV vectors; transplanting naturally occurring HIV-resistant hematopoietic stem cells; and enrolling appropriate HIV-infected patients alongside HIV− lymphoma patients in clinical trials.
Acknowledgment
Editorial support in the preparation of this manuscript was provided by Hannah Rice.
Authorship
Contribution: A.N. researched and wrote the manuscript as the sole author.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Ariela Noy, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: noya@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal