Abstract
Allogeneic hematopoietic cell transplantation involves consideration of both donor and recipient characteristics to guide the selection of a suitable graft. Sufficient high-resolution donor–recipient HLA match is of primary importance in transplantation with adult unrelated donors, using conventional graft-versus-host disease prophylaxis. In cord blood transplantation, optimal unit selection requires consideration of unit quality, cell dose and HLA-match. In this summary, the National Marrow Donor Program (NMDP) and the Center for International Blood and Marrow Transplant Research, jointly with the NMDP Histocompatibility Advisory Group, provide evidence-based guidelines for optimal selection of unrelated donors and cord blood units.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 994.
Disclosures
Author Miguel-Angel Perales serves as a member of the scientific advisory board for MolMed S.p.A. and NexImmune Inc.; received honoraria from AbbVie Inc., Bellicum Pharmaceuticals, Inc., Incyte Corporation, Medigene AG, Merck & Co., Inc., Nektar Therapeutics, Novartis Pharmaceuticals Corporation, and Servier; and received research funding from Incyte Corporation and Miltenyi Biotec. Associate Editor Robert Zeiser, CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, and the remaining authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe updated evidence-based recommendations from the National Marrow Donor Program and the Center for International Blood and Marrow Transplant Research (NMDP/CIBMTR) regarding selection of unrelated donors for hematopoietic cell transplantation (HCT)
Determine updated evidence-based recommendations from NMDP/CIBMTR regarding selection of umbilical cord blood units for HCT using optimal HLA donor-recipient matching criteria and other factors affecting graft selection
Identify updated evidence-based recommendations from NMDP/CIBMTR regarding adult donor search, optimal HLA donor-recipient matching criteria and other factors affecting graft selection
Release date: September 19, 2019; Expiration date: September 19, 2020
Introduction
The National Marrow Donor Program (NMDP) facilitates the identification and procurement of unrelated donor grafts for hematopoietic cell transplantation (HCT). The Center for International Blood and Marrow Transplant Research (CIBMTR) is a research collaboration between the NMDP and the Medical College of Wisconsin. The guidelines below created jointly with the NMDP Histocompatibility Advisory Group, which consists of key opinion leaders in immunogenetics and HCT, update those previously published in 2003,1 2008,2 and 20123 and are based on current and relevant data supporting optimal HLA donor–recipient matching criteria and other factors affecting graft selection.
The majority of our recommendations in this review are based on the existing precedent of overall survival (OS) as the primary outcome of interest. While this is an unambiguous measure of success, we note that composite end points such as graft-versus-host disease (GVHD), relapse-free survival as well as patient-reported outcome measures such as quality of life provide valuable information on HCT outcome beyond survival status. While we acknowledge that multiple pre- and posttransplant patient, disease, and transplantation variables impact outcome, this review focuses primarily on the impact of HLA and non-HLA factors considered in donor selection pretransplant.4,,,,,,,,,,,,,,,,,-22 Disease stage is a major determinant of transplant outcome, and prompt transplantation is optimal for patients with high-risk disease.21,23 Rapid assessment of the likelihood of unrelated donor search success, clear guidance provided by physicians to their search coordinators, and prioritization of alternative donor strategies are needed to avoid delay associated with futile unrelated donor searches.24 Since not all patients will have a fully HLA-matched donor,25 it is important to consider whether such patients should pursue an HCT from an alternative donor source such as cord blood (CB) or haploidentical related or mismatched unrelated donor. We note that these all represent viable alternative donor options. In the absence of definitive comparative outcome data, we do not recommend one as a preferred approach. Individual-patient– (disease risk, urgency in time to HCT, and donor availability) and provider-level considerations influence selection.
HLA typing
Definition of high resolution
Nomenclature for HLA is described at http://hla.alleles.org.26,27 DNA-based nomenclature has a potential of 4 numerical fields separated by colons (eg, A*02:01:01:01). “Allele”-level typing, also termed high-resolution typing or 2-field typing,28 discriminates among HLA genes that encode cell-surface proteins that differ in the amino acid sequence of their antigen recognition domain (ARD).29 Other designations that indicate ARD identity include G (A*02:01:01G indicating nucleotide sequence identity in the ARD exons) and P (A*02:01P indicating protein sequence identity in the ARD) nomenclature.26 Both G and P groups of alleles can also be represented as multiple allele codes that are provisioned globally by the NMDP. A description of the alleles included in a specific code can be found at https://hml.nmdp.org/MacUI/. The ARD is the “active” portion of the HLA molecule that binds peptide antigens and interacts with T lymphocyte and natural killer cell receptors. Available data suggest that alleles that are identical in the ARD but differ in amino acid sequence in other regions of the protein do not stimulate direct allorecognition, but this needs to be evaluated in a retrospective large-scale study of outcome.30 Consequently, HLA reports may designate a donor or recipient as having one of several possible alleles, all with the same ARD, for a given locus and it is standard practice to accept identity of these donor and recipient assignments as a match. A list of alleles that encode the same amino acid sequence in the ARD can be found at http://hla.alleles.org.
HLA typing recommendations for patients and adult donors
Patients and donors should be typed by DNA-based methods at high resolution for HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DPB1 loci.31 Other loci (eg, HLA-DQB1, HLA-DRB3/4/5, HLA-DQA1, and HLA-DPA1) have not been shown in isolation to substantially impact survival; however, they may assist in designing an efficient search strategy for the patient and, when possible, for selecting among multiple similar donors and support donor selection in the context of an HLA-sensitized patient to avoid the potential risk of graft failure.32,33
HLA typing recommendations for umbilical CB units
CB units should be typed by DNA-based methods at high resolution for HLA-A, HLA-B, HLA-C, and HLA-DRB1.9 The NMDP centralized confirmatory typing program performs high-resolution typing and also includes DQB1 and DPB1.
Selection of adult unrelated donors
HLA matching considerations
Optimal match criteria for unrelated adult donors
HLA-mismatching between donors and recipients in the setting of conventional GVHD prophylaxis is consistently associated with inferior recipient survival in both the myeloablative and the reduced-intensity conditioning context, as well as in the setting of T-cell–replete and T-cell–depleted grafts.5,21,23,34,35
Results apply to both bone marrow (BM) and peripheral blood stem cell (PBSC) grafts.21,23,36 Although the majority of studies have been performed in patients receiving HCT for malignant diseases, there is supporting data for a similar adverse impact on survival in patients with nonmalignant disorders.37,38
A recent analysis from the NMDP/CIBMTR affirmed major findings from prior studies, refined donor selection guidelines, and validated the importance of avoiding nonpermissive DPB1 mismatches (described in “Variation in HLA protein structure”) to optimize survival.21 This analysis included adult and pediatric recipients of first myeloablative unrelated donor BM or PBSC transplant for acute myelogenous leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia, or myelodysplastic syndrome between 1999 and 2011. Importantly, the study population more closely approximated current HCT practices compared with prior analyses, given the greater representation of PBSCs as a graft source and use of non–total body irradiation–containing myeloablative conditioning regimens. All cases had high-resolution typing for HLA-A, HLA-B, HLA-C, and HLA-DRB1, while a subgroup had typing available for analysis of the effect of mismatch at DPB1 and DQB1. Of the total (n = 8003), cases were 8/8 HLA-matched (n = 5449), 7/8 (n = 2071), or 6/8 (n = 483) matched at HLA-A, HLA-B, HLA-C, and HLA-DRB1. Several major findings from this study informed current donor selection guidelines (Table 1): First, any single mismatch at HLA-A, HLA-B, HLA-C, or HLA-DRB1 was associated with significantly worse OS, and there was no evidence that mismatch at any of the individual loci was better tolerated than others. Multiple mismatches worsened OS further, supporting that 6/8 or less-matched transplants do not provide acceptable OS outcome under conventional HCT practices represented in that study. In addition, there was no difference in the impact of mismatch on OS according to allele-level vs antigen-level mismatch, supporting a need to match at the allele level. The effect of HLA mismatch differed according to disease risk, as the greatest negative impact on OS was observed for those with early-stage disease. Among 8/8 matched cases, DPB1 and DQB1 mismatch resulted in increased acute GVHD, but DPB1 mismatch decreased relapse risk. Building from preclinical observations regarding the immunogenicity of specific HLA-DPB1 alleles,39,,-42 this analysis also confirmed the adverse impact of nonpermissive HLA-DPB1 mismatch (described in “Variation in HLA protein structure”) on OS.21 Similar results were obtained in other large studies.41,43,44 The directionality of nonpermissive HLA-DPB1 mismatches does not appear to alter this impact.45
Guidelines for unrelated donor selection
| . | Multiple HLA-A, HLA-B, HLA-C, and HLA-DRB1 (8/8) HLA matched unrelated donors available . | 8/8 match unavailable; multiple 7/8 unrelated donors available . |
|---|---|---|
| 1. Resolution of typing HLA-A, HLA-B, HLA-C, and HLA-DRB1 | High-resolution, matches for ARDs | High-resolution matches for ARDs for 7 matched alleles; |
| Select HLA-C*03:03 vs C*03:04 mismatch, if present; | ||
| No other preference for mismatched loci (HLA-A/B/C/DRB1) or other allele combinations | ||
| 2. Donor age | Select donors of younger age | Select donors of younger age |
| 3. Permissive mismatching HLA-DPB1 | Select matched/permissive DPB1 mismatch based on the algorithm developed by Crivello et al68,70 (http://www.ebi.ac.uk/cgi-bin/ipd/imgt/hla/dpb_v2.cgi) | Select matched/permissive DPB1 mismatch based on the algorithm developed by Crivello et al68,70 (http://www.ebi.ac.uk/cgi-bin/ipd/imgt/hla/dpb_v2.cgi) |
| 4. Matching HLA-DRB3/4/5 and HLA-DQB1 | Minimize mismatches | Minimize mismatches |
| 5. Vector of mismatch | N/A | Select donor with single allele mismatched at patient’s homozygous locus (HLA-A/B/C/DRB1), if applicable |
| 6. DSA in patient | Avoid mismatches of allotypes targeted by DSAs, including DQA1 and DPA1 | Avoid mismatches of allotypes targeted by DSAs, including DQA1 and DPA1 |
| 7. Transplant center practice may differ in additional considerations to use in the selection among multiple donors equivalent for the characteristics above | ||
| . | Multiple HLA-A, HLA-B, HLA-C, and HLA-DRB1 (8/8) HLA matched unrelated donors available . | 8/8 match unavailable; multiple 7/8 unrelated donors available . |
|---|---|---|
| 1. Resolution of typing HLA-A, HLA-B, HLA-C, and HLA-DRB1 | High-resolution, matches for ARDs | High-resolution matches for ARDs for 7 matched alleles; |
| Select HLA-C*03:03 vs C*03:04 mismatch, if present; | ||
| No other preference for mismatched loci (HLA-A/B/C/DRB1) or other allele combinations | ||
| 2. Donor age | Select donors of younger age | Select donors of younger age |
| 3. Permissive mismatching HLA-DPB1 | Select matched/permissive DPB1 mismatch based on the algorithm developed by Crivello et al68,70 (http://www.ebi.ac.uk/cgi-bin/ipd/imgt/hla/dpb_v2.cgi) | Select matched/permissive DPB1 mismatch based on the algorithm developed by Crivello et al68,70 (http://www.ebi.ac.uk/cgi-bin/ipd/imgt/hla/dpb_v2.cgi) |
| 4. Matching HLA-DRB3/4/5 and HLA-DQB1 | Minimize mismatches | Minimize mismatches |
| 5. Vector of mismatch | N/A | Select donor with single allele mismatched at patient’s homozygous locus (HLA-A/B/C/DRB1), if applicable |
| 6. DSA in patient | Avoid mismatches of allotypes targeted by DSAs, including DQA1 and DPA1 | Avoid mismatches of allotypes targeted by DSAs, including DQA1 and DPA1 |
| 7. Transplant center practice may differ in additional considerations to use in the selection among multiple donors equivalent for the characteristics above | ||
DSA, donor-specific HLA antibodies.
Criteria for the best partially matched unrelated donor
Although multiple algorithms for selecting permissive or tolerated mismatches have been proposed (eg, location and characteristics of amino acid mismatches),46,,,,,,,,-55 most have failed to be validated in large data sets.56 The immunologic impact of HLA mismatches is thought to be contingent upon 3 major factors: (1) effect of the mismatch on the physical and chemical structure of the HLA molecule and its bound peptide53 ; (2) direction of the mismatch, either in the GVHD vector or in the host-versus-graft direction (HvG) vector or bidirectional57 ; and (3) level of expression of the mismatched HLA molecule on the cell surface.58,59
Variation in HLA protein structure
One example of an acceptable mismatch is the HLA-C*03:03 vs HLA-C*03:04 allele combination. HLA-C*03:03/03:04 is the most frequent HLA-C mismatch in individuals of European ancestry, and both alleles are associated with B*15:01.60 These 2 HLA proteins differ subtly from one another and do not appear to result in allorecognition.61,-63 In a retrospective study, 7/8 matched pairs with the HLA-C*03:03/03:04 mismatch had similar outcomes (mortality, disease-free survival, grade 3-4 acute GVHD) to 8/8 matched pairs.64
Due to weak linkage disequilibrium with other HLA loci and lack of historic typing available on donor registries, DPB1 mismatches were shown to be present in >80% of unrelated HCT.17 Since DPB1 matches are less common and typing less frequently available at initial search, searches should not focus on identifying a DPB1 matched donor. Rather, the goal is to generate limited alloreactivity needed for a graft-versus-leukemia effect while preventing excessive alloreactivity associated with acute GVHD. Therefore, differentiation between low-risk DPB1 mismatches and high-risk DPB1 mismatches is clinically important.65 One model to predict level of immunogenicity is based on the ability of specific DP allotypes to induce T-cell alloreactive responses.66 Three T-cell epitope (TCE) groups41,67 differing by the strength of their immunogenicity are assigned; most recently, these epitope groups have been expanded to include more DP allotypes based on amino acid sequence similarities.68,-70 TCE matching is available in the NMDP Traxis Web-based software application for transplant centers and a publically available tool (http://www.ebi.ac.uk/ipd/imgt/hla/dpb_v2.html). While allele matching at DPB1 is less frequent, identifying TCE permissive donors can be highly successful.71
Direction of the mismatch
If either the recipient or donor expresses only a single HLA allele at a locus (ie, is homozygous), then a unidirectional mismatch will occur. There are 2 types of unidirectional mismatches in HCT. If the recipient is homozygous at a specific locus, including presence of a null allele, and the donor is heterozygous and mismatched at that particular locus, then the mismatch is in the HvG vector. If, in contrast, the donor is homozygous at a specific HLA locus and the recipient is heterozygous at the same locus, then the mismatch is in the graft-versus-host vector. When both recipient and donor are homozygous and mismatched at that locus or when both are heterozygous with a 7/8 match, then the mismatch is bidirectional.
A retrospective multicenter study found a significantly lower risk of acute GVHD in the 7/8 HvG group compared with 7/8 graft-versus-host and bidirectional mismatched groups.57 However, no difference was observed on OS between unidirectional or bidirectional mismatches and all carried a higher risk of death compared with an 8/8 match.
Matching consideration of expression level of HLA loci
Impact of HLA mismatches on allorecognition is dependent on the ability of donor T lymphocytes to detect foreign HLA on the surface of cells. HLA loci vary in their level of expression on the surface of the cells, and these differences may affect allorecognition and impact transplant outcomes.59,72 A study of registry data with predominantly BM transplant recipients demonstrated significant lower OS and increased transplant related mortality in 7/8 unrelated donor cohort with ≥3 mismatches in low expression HLA loci, HLA-DRB3/4/5, DQB1, and DPB1. While mismatches at these loci do not appear to impact outcomes in the 8/8 cohort, it is recommended that matching in the 7/8 situation consider these secondary loci.73
Matching requirements for BM and PBSCs
Currently, most unrelated adult donor transplantations use granulocyte colony-stimulating factor mobilized PBSC grafts. We acknowledge that a major randomized trial demonstrated increased risk of chronic GVHD,76 as well as long-term impairment in quality of life when PBSC grafts are used rather than BM in unrelated donor HCT.77 A prior CIBMTR analysis of HLA matching in 1933 unrelated PBSC transplantations for hematologic malignancies36 supported adverse impact of HLA mismatch, providing important new information given the predominance of BM grafts in prior studies. A more recent study that included >8003 donor–recipient pairs demonstrated that, irrespective of graft type, HLA mismatch (6-7/8 vs 8/8) was significantly associated with increased acute GVHD, chronic GVHD, treatment-related mortality, and OS. Graft type had no impact on OS during the first year after transplant, but beyond 1 year, PBSC grafts were associated with increased mortality risk.21
Consideration of non-HLA factors
Impact of donor availability
More than 33 million people are currently registered worldwide as potential donors (www.wmda.info). Most are not pursued as potential matches until months or years after initially volunteering. Historically, NMDP found that nearly 50% of registered donors were unavailable when identified as a potential donor because of changes in their personal circumstances or inability to contact them.78 Donor availability rates differ by international registry and by donor race/ethnic groups, adding to the challenge particularly for ethnically diverse populations. New strategies of engagement have resulted in dates of last contact and recommitment being included in search reports to aid transplant centers in selecting donors with a higher likelihood (>70%; unpublished internal NMDP data) of being available. However, it is still important that centers pursue multiple adult donors or suitable CB units, as other issues such as donor medical fitness may impact the timeliness of the search and donation process.
Consideration of NK cell alloreactivity
Guidelines for selection of an unrelated donor to maximize the activation of natural killer (NK) cells to deliver an antileukemic effect and to improve survival continue to be elusive.79,-81 Many factors appear to control donor-derived NK cell activity, including the donor’s killer cell immunoglobulin-like receptor (KIR) gene content,82,83 HLA class I ligands expressed by the recipient,84,,-87 licensing of NK cells in the recipient,88 donor–recipient matching for KIR genotypes,89 graft source,84 conditioning regimen,90 and the presence of NK cell activating ligands on malignant cells.84,87 Deliberate selection of an HLA-mismatched donor to activate NK cells is not supported by current data, so identifying an HLA-matched donor remains the first priority. Adult donor selection based on KIR should currently only be considered within the context of a clinical trial or center-specific practice.
Impact of nongenetic donor characteristics
While HLA matching remains the primary criteria for donor selection, non-HLA factors are often considered when selecting donors, including cytomegalovirus serostatus, sex, age, ABO compatibility, prior pregnancies, and larger body weight. In a recent large study that specifically addressed donor characteristics, the only characteristic associated with OS was donor age; recipient mortality was higher with increasing donor age.5 Donor age was also the only characteristic associated with OS in a study attempting to validate a donor selection score.91 Logistical issues may determine the selection between these secondary donor characteristics if multiple equally matched and age donors are available.
Impact of race/ethnicity in the selection process
Many HLA alleles and haplotypes are distributed at different frequencies among different racial/ethnic groups.25,92,93 HLA alleles at low frequency in the general population are more likely found on distinct haplotypes along with the remainder of the HLA alleles from an ancestral racial/ethnic group in common between patient and donor. NMDP’s predictive matching algorithm, HapLogic, takes the race/ethnic group into account when predicting the likelihood of a high-resolution match, so centers should attempt to accurately obtain and enter these patient details for the search.
Consideration of patient sensitization
Approximately one-third of adults with hematologic malignancies are sensitized to HLA antigens as demonstrated by the presence of circulating antibodies.33 The incidence of humoral sensitization against HLA is 2 to 3 times higher in females than in males and increases with the number of pregnancies. Although several studies have shown a strong association of preformed donor-specific HLA antibodies (DSA) with primary graft failure after unrelated donor transplantation32,33 and related haploidentical donor transplantation,94,95 the data in the setting of CB transplants are mixed, with some reporting an adverse effect and others none.96,,-99 Thus, for patients with anti-HLA antibodies and a mismatched allograft, careful antibody specificity analysis and/or testing of the patient’s serum for reactivity with cells from potential donors (ie, cross-matching) should be done prior to transplantation and the threshold determined by local laboratory standards. It should be noted that the specificity of some DSA results from the recognition of epitopes determined by the DQA1, DPA1 or present in some loci (DRB3/4/5, DQ, DP) not included in the protocols to define the match grade between the patient and the selected donor. The presence of these antibodies should prompt further HLA testing of these loci. When DSA is identified, the most straightforward choice to reduce the risk for HLA antibody–mediated graft failure is to select donors with mismatched alleles that are not the target of DSA. If no HLA antibody–compatible donors can be identified, desensitization treatment of reducing the levels or eliminating DSA before transplantation may improve the chances of successful donor engraftment.94
Selection of umbilical CB units
Non-HLA criteria: unit quality and cell dose
In HCT with CB, unlike adult volunteer donors, non-HLA factors are as critical as HLA matching in unit selection. CB has the major advantage of rapid availability and a markedly reduced stringency of HLA-matching compared with adult unrelated PBSC or BM. Consequently, use of CB has dramatically extended allograft access to racial and ethnic minorities.9,25,100 Recent experience in CB transplantation has demonstrated improving rates of sustained donor engraftment.101,102 These result from strategies to abrogate graft rejection combined with optimal CB graft selection. Optimal CB graft selection criteria must consider unit quality and cryopreserved cell dose9,103,104 as well as HLA match. They have recently been reviewed in Barker et al on behalf of the NMDP and the American Society for Blood and Marrow Transplantation (ASBMT) CB Special Interest Group.9 Unit quality results from banking practices. It has increasingly been recognized as a critical factor in unit selection (factors outlined in Table 2). This is because unit quality is highly associated with unit potency, including postthaw CD34+ cell viability and recovery.101,105
Unrelated CB unit selection guidelines
| . | Guidelines . |
|---|---|
| Bank practices | |
| Attached segment identity testing | Mandatory |
| Use of RBC-replete units*† | Not recommended |
| Cryovolume‡ | Should be considered, especially if the unit is to be diluted post thaw |
| Year of cryopreservation | More recent units may be linked to optimal banking practices depending on the bank |
| Bank location | Domestic or international units fulfilling selection criteria |
| Bank accreditation and/or licensure | Should be considered |
| HLA match | |
| Resolution of HLA typing | Minimum of 8 high-resolution (HLA-A, HLA-B, HLA-C, and HLA-DRB1) for both patient and CB unit |
| Donor–recipient HLA match | ≥4/6 HLA-A and HLA-B antigen, HLA-DRB1 high-resolution (traditional match), and ≥4/8 high-resolution match (some centers investigating use of 4/6 and 3/8 units if adequate dose) |
| Unit–unit HLA match for double unit CBT | Not required |
| Avoidance of units against which recipient has DSA§ | Conflicting results in hematological malignancies; avoid if nonmalignant diagnosis |
| Cryopreserved cell dose||¶# | |
| Single-unit CBT: minimum dose/kg | TNC ≥2.5 × 107/kg and CD34+ cells ≥1.5 × 105/kg (some centers recommend higher CD34+ dose as minimum) |
| Double-unit CBT: minimum dose/kg per unit | TNC 1.5 × 107/kg for each unit and CD34+ cells ≥1.0 × 105/kg for each unit (some centers recommend higher CD34+ doses for each unit as minimum) |
| . | Guidelines . |
|---|---|
| Bank practices | |
| Attached segment identity testing | Mandatory |
| Use of RBC-replete units*† | Not recommended |
| Cryovolume‡ | Should be considered, especially if the unit is to be diluted post thaw |
| Year of cryopreservation | More recent units may be linked to optimal banking practices depending on the bank |
| Bank location | Domestic or international units fulfilling selection criteria |
| Bank accreditation and/or licensure | Should be considered |
| HLA match | |
| Resolution of HLA typing | Minimum of 8 high-resolution (HLA-A, HLA-B, HLA-C, and HLA-DRB1) for both patient and CB unit |
| Donor–recipient HLA match | ≥4/6 HLA-A and HLA-B antigen, HLA-DRB1 high-resolution (traditional match), and ≥4/8 high-resolution match (some centers investigating use of 4/6 and 3/8 units if adequate dose) |
| Unit–unit HLA match for double unit CBT | Not required |
| Avoidance of units against which recipient has DSA§ | Conflicting results in hematological malignancies; avoid if nonmalignant diagnosis |
| Cryopreserved cell dose||¶# | |
| Single-unit CBT: minimum dose/kg | TNC ≥2.5 × 107/kg and CD34+ cells ≥1.5 × 105/kg (some centers recommend higher CD34+ dose as minimum) |
| Double-unit CBT: minimum dose/kg per unit | TNC 1.5 × 107/kg for each unit and CD34+ cells ≥1.0 × 105/kg for each unit (some centers recommend higher CD34+ doses for each unit as minimum) |
Developed by the ASBMT CB Special Interest Group. For successful engraftment, optimal CB graft selection and the patient’s rejection risk must be considered.9
CBT, CB transplant; RBC, red blood cell; TNC, total nucleated cell.
RBC-replete units have been associated with life-threatening infusion reactions. Washing is difficult due to the lack of a clear interface after centrifugation; washing also risks cell loss. Therefore, RBC-replete units should be used with caution. They should only be considered in the absence of RBC-depleted CB units meeting acceptable criteria.
Incorporation of nucleated red cell content in unit selection is not recommended at this time.
Some expert centers prefer to use an RBC-depleted unit that has a post-cryopreservation volume of ∼25 mL/bag. If a unit was divided into 2 bags for storage, then each bag should contain ∼25 mL.
Regarding the significance of HLA antibodies, DSAs must be considered on a case-by-case basis based on diagnosis and prior immunosuppressive therapy that determine rejection risk, the intensity of planned conditioning, and the number, titer, specificity, and complement fixation of DSAs. DSA targeted units should be avoided in nonmalignant diagnoses. In patients with malignancies, avoid if possible, but use caution if avoidance of units against which the patient has antibodies compromises the selected CB unit dose and HLA match.
For single- vs double-unit CB transplant, if no adequate single-unit graft is available, then a double-unit graft is recommended. Clinical trials investigating the addition of other cellular products to a single-unit graft can also be considered.
For prioritization of cell dose vs HLA match (applies to single- and double-unit transplants), cell dose frequently needs to take priority over HLA match for adult and larger pediatric patients. HLA-match can take priority in children or smaller adults or those with common HLA typing who have multiple units with high cell dose. Optimizing HLA-match is very important in CB transplant for nonmalignant diagnoses. In children with nonmalignant diagnoses, higher cell doses (≥5 × 107/kg) should be selected. Further data are required as to how to balance cell dose against HLA match. A current guidance for consideration is as follows: if high doses (eg, TNC ≥3 × 107/kg and CD34+ ≥2 × 105/kg), consider optimizing high-resolution HLA match over cell dose; if lower TNC and CD34+ doses, optimize dose first and high-resolution HLA match second; and if units have similar cell doses, optimize high-resolution HLA match.
Reporting of unit viability testing is not fully standardized. Flow-based assays of CD34+ cell viability on a segment can be informative but have not been validated in multiple banks/centers. The NMDP will facilitate discussion between centers and the bank if questions concerning viability testing arise.
In a collaborative analysis by the CIBMTR, Eurocord and the European Group for Blood and Marrow Transplantation, TNC doses of ≥3.0 × 107/kg recipient body weight for single units was associated with sufficient progenitor cells for successful transplantation.106 Increasing TNC dose beyond 3.0 × 107/kg did not confer an advantage in regards to hematopoietic recovery or survival, but lower TNC was associated with higher mortality. In the Blood and Marrow Transplant Clinical Trial Network (BMT CTN 0501) trial comparing single vs double CB transplantation, an adequately dosed single unit was defined as having a minimum TNC dose of ≥2.5 × 107/kg (while mean TNC doses in this trial were higher, both for single CB units, and each CB unit in the setting of double CB transplants).107,108 In the absence of an adequately dosed single unit, infusion of 2 units is the standard, with each unit containing a minimum TNC of 1.5 × 107/kg. Experienced CB transplant programs now consider CD34+ progenitor content a better measure of unit potency, and incorporation of CD34+ dose in unit selection is now considered standard practice.9,101 Minimum prefreeze CD34+ of 1.5 × 105/kg for single unit or 1.0 × 105/kg for each unit when infusing 2 units is desirable (Table 2). Caution is warranted when there are disparities between the CD34+ dose and the TNC dose (ie, one is high and the other is low) that may reflect a laboratory or unit report listing error.
HLA matching requirements for umbilical CB units
While the historical standard for selecting unrelated CB has been based on HLA-A, HLA-B antigen (ie, first field typing equivalent to a serologic match) and HLA-DRB1 allele (ie, high-resolution or 2-field match), the available data in 20123 supported the inclusion of the HLA-C antigen in the matching calculation to minimize mortality risks.109 Later reports confirmed the importance of counting matches at the high-resolution level for HLA-A, HLA-B, HLA-C, and HLA-DRB1 for malignant and nonmalignant diseases.106,110 The incidence of neutrophil recovery was lower and graft failure and mortality rates were higher when the recipient and the CB unit were mismatched at ≥2 HLA high-resolution types. In the setting of double CB unit transplantation, the same HLA match criteria that guide single-unit selection should be applied to the selection of both units.111 There are no data that support consideration of interunit HLA matching in double-unit graft selection.101,112,113 Taken together, guidelines have been developed by the ASBMT CB Special Interest Group (summarized in Table 2). Overall, the current data support selecting units using the following unit principles: (1) adequate unit quality, (2) minimum required TNC and CD34+ cell doses, and (3) optimal HLA high-resolution matched unit considering HLA-A, HLA-B, HLA-C, and HLA-DRB1. To date, there are no data to further guide how to balance CD34+ dose against HLA high-resolution match grade. Further study of this question is required and recommendations may differ for single- vs double-unit grafts. A general guidance in this evolving field is provided in Table 2.
Adult donor search
Search strategy
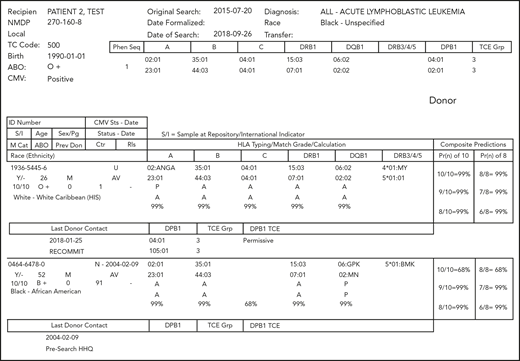
Consistent with the recommended HLA typing for the patient, the search should be based on high-resolution HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DPB1, with additional loci (eg, HLA-DQB1 and DRB3/4/5) optional but often helpful in designing an efficient search strategy. Although the practice of many registries in recent years is to high-resolution type new donors at recruitment, the majority of worldwide donors do not have extended high-resolution typing of all of these loci available. The NMDP search algorithm, HapLogic, leverages data on the frequencies of alleles and haplotypes defined by ethnic populations to predict the probability of high-resolution matches at individual HLA loci and at all key loci simultaneously (Figure 1) for the patient and each potential donor.114,115
Example of an NMDP search report. HLA assignments shown include high-resolution/2-field assignments (eg, A*02:01) and the use of multiple allele codes (eg, A*02:ANGA).115 These codes indicate that the assignment has not discriminated among ≥2 alternative alleles. A description of the alleles included in a specific code can be found at https://hml.nmdp.org/MacUI/. The columns labeled “HLA Typing/Match Grade/Calculation” use a letter indicating the match status of each allele at the locus (A indicates allele match; P, potential allele match; and M, mismatch) and the probability of matching both alleles at the locus (99% for the first donor at each locus). The columns labeled “Composite Predictions” provide the probability of a 10 of 10 HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 allele match (99% for the first donor) and 8 of 8 HLA-A, HLA-B, HLA-C, and HLA-DRB1 allele match (99% for the first donor). Donor DPB1 alleles and TCE group designations are provided when the donor is typed at DPB1, and DPB1 TCE Match Assignments (“DPB1 TCE”) are provided when both donor and patient are typed at DPB1. Non-HLA donor demographics are also displayed, including donor age, gender, blood type, cytomegalovirus serostatus, number of prior pregnancies, and last date the registry had contact with the donor (“Last Donor Contact”).
Example of an NMDP search report. HLA assignments shown include high-resolution/2-field assignments (eg, A*02:01) and the use of multiple allele codes (eg, A*02:ANGA).115 These codes indicate that the assignment has not discriminated among ≥2 alternative alleles. A description of the alleles included in a specific code can be found at https://hml.nmdp.org/MacUI/. The columns labeled “HLA Typing/Match Grade/Calculation” use a letter indicating the match status of each allele at the locus (A indicates allele match; P, potential allele match; and M, mismatch) and the probability of matching both alleles at the locus (99% for the first donor at each locus). The columns labeled “Composite Predictions” provide the probability of a 10 of 10 HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 allele match (99% for the first donor) and 8 of 8 HLA-A, HLA-B, HLA-C, and HLA-DRB1 allele match (99% for the first donor). Donor DPB1 alleles and TCE group designations are provided when the donor is typed at DPB1, and DPB1 TCE Match Assignments (“DPB1 TCE”) are provided when both donor and patient are typed at DPB1. Non-HLA donor demographics are also displayed, including donor age, gender, blood type, cytomegalovirus serostatus, number of prior pregnancies, and last date the registry had contact with the donor (“Last Donor Contact”).
An NMDP search includes donors from all registries that list their donors in the World Marrow Donor Association Search and Match listing (previously known as Bone Marrow Donors Worldwide) via download and direct electronic connections.116 Transplant centers can use the World Marrow Donor Association listing or the listing of their selected registry, if also globally comprehensive, for the evaluation of donors and CB units. Most registries provide free patient preliminary searches to member centers.
The optimal number of potential donors to select from the search report for additional HLA typing should be individualized for each patient since many factors influence the likelihood of finding a compatible donor. Considerations include the patient’s alleles and haplotypes (eg, rare vs common), ethnic background of the donor options, and clinical urgency. Multiple donors should always be selected, because donors may be unavailable, medically unsuitable to be donors either for donor or recipient safety, mistyped, or not matched once high-resolution testing is complete.
Whenever deemed useful, the NMDP can provide lists with center-defined matching criteria that allow for multiply mismatched donor sources not typically shown on traditional unrelated donor matching algorithms. Consultation with a histocompatibility expert is available through the NMDP to design an effective search strategy that includes evaluation of worldwide donor registries.
Acceptable search time range and evaluating unrelated donor search futility
For patients with fairly common HLA genotypes, a suitably matched adult donor can be quickly identified in most cases, often upon first review of the search results. Early evaluation of patient search difficulty can provide vital information for determining a clinical treatment and selection strategy, including which donor sources should be pursued, how many donors/CB units may be required to identify a match, and how to achieve the needed transplant timeline.24,117 For patient searches that are more difficult, such as those with no (or just a few) donor candidates or those with a large number of donors with low probability to match the patient, transplant centers should establish an acceptable time limit to spend on donor search while initiating concurrent activities for other acceptable options such as CB and mismatched related or unrelated donor options. Table 1 provides key recommendations for selection of 8/8 matched unrelated donors, as well as considerations when selecting 7/8 matched unrelated donors. In the absence of 8/8 matched unrelated donors, we acknowledge that 7/8 matched unrelated donors, umbilical CB, and related haploidentical donors all represent viable options, and that selection will depend on patient- and provider-level factors.
Importantly, the NMDP HapLogic donor search considers over 20 million donors (87% typed for HLA-A, HLA-B, and HLA-DR) and additionally provides a match report of an additional 13 million donors listed in World Marrow Donor Association Search and Match. Therefore, patients who are not able to find a suitably matched donor in this pool have uncommon or rare HLA genotypes. Given the NMDP adds an average of 30 000 new donors to the file monthly and the rest of the world adds ∼150 000, the likelihood that a patient’s type will be represented in those new recruits for the first time is very low. Therefore, for patients requiring transplant, waiting for a match is not recommended.
New strategies have recently been developed that can accurately predict search prognosis based on the patient’s typing or a patient search prognosis categorization derived from the HapLogic match predictions of each unrelated donor.24,117 A search prognosis tool based on a patient’s HLA typing commonality is available online (http://search-prognosis.b12x.org). These algorithms can be used at search initiation to efficiently triage patients to pursuing alternative donors when the unrelated donor search is poor or futile.
Conclusion
The field of HCT continues to be guided by research evidence that evolves our understanding of how clinical patient care should be adapted to ensure the best clinical outcomes. This work updates prior guidelines based on more recent research studies to identify minimum considerations for HCT practices evaluated in concert with the NMDP histocompatibility advisory group.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service grant/cooperative agreement 5U24CA076518 from the National Institutes of Health, National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; grant/cooperative agreement 1U24HL138660 from the National Heart, Lung, and Blood Institute and National Cancer Institute; contract HHSH250201700006C with the Health Resources and Services Administration (Department of Health and Human Services), 3 grants (N00014-17-1-2388, N00014-17-1-2850, and N00014-18-1-2045) from the Office of Naval Research; and grants from Adaptive Biotechnologies; *Amgen, Inc.; an anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol-Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; *CytoSen Therapeutics, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell, Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; *Kite Pharma, Inc.; Medac, GmbH; *Mediware; the Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast, MesoScale Diagnostics, Inc.; Millennium; the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; Mundipharma EDO; the NMDP; Novartis Pharmaceuticals Corporation; PCORI; *Pfizer, Inc.; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; *Takeda Oncology; and the University of Minnesota. *Corporate Members
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: J.D., S.S., C.K.H., B.E.S., J.N.B., L.J.B., D.L.C., M.E., M.F.-V., R.H., M.M., S.R.M., C.M., M.-A.P., R.R., and J.P. wrote the paper. All authors approved the manuscript and submission.
Conflict-of-interest disclosure: M.-A.P. serves as a member of the scientific advisory board for MolMed and NexImmune and has received honoraria from AbbVie, Bellicum, Incyte, Medigene, Merck, Nektar Therapeutics, Novartis, and Servier and research funding from Incyte and Miltenyi. The remaining authors declare no competing financial interests.
Correspondence: Jason Dehn, National Marrow Donor Program, 500 N 5th St, Minneapolis, MN 55401; e-mail: jdehn@nmdp.org.