In this issue of Blood, Zheng et al provide insight into the source of intravascular tissue–type plasminogen activator (tPA). They demonstrate an important role for hepatocytes in determining the basal level of plasma tPA and identify regulatory factors that control tPA expression by hepatocytes.1
The plasma level of tPA reflects contributions from vascular endothelium and hepatocytes. Hepatocyte expression of tPA is regulated by DACH1 and ATF6.
The plasma level of tPA reflects contributions from vascular endothelium and hepatocytes. Hepatocyte expression of tPA is regulated by DACH1 and ATF6.
Decades ago, it became apparent that plasminogen activators can be detected in human plasma at a basal level and at substantially increased levels in response to vascular injury, stasis, or occlusion.2 On the basis of the methods used to detect plasminogen activators, it was obvious that the source of the proteinase released into the plasma in response to stasis was the blood vessel wall. Subsequent work demonstrated that the major plasminogen activator released by the vessel wall was tPA, that the cellular source of releasable tPA was mainly endothelial cells, and that plasminogen activator activity observed in plasma samples reflects a balance between tPA and inhibitors in the serpin family such as plasminogen activator inhibitor-1.3,4
The release of tPA into the blood in response to vascular injury provides a checkpoint for coagulation, which prevents unnecessary vascular occlusion under challenging conditions. Clearly, the amount of tPA present in any region of the vasculature at any time reflects the basal level of tPA in that patient and tPA released locally by endothelium in response to various stimuli. Interestingly, the source of constitutively expressed plasma tPA under basal conditions has never been extensively explored.
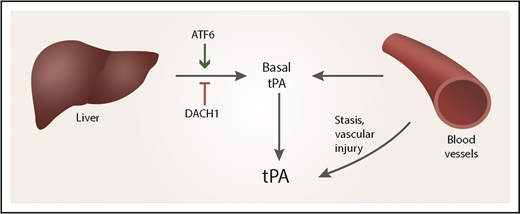
Zheng et al began their work by obtaining a transcriptome profile of hepatocytes in which the gene encoding the transcriptional corepressor, DACH1, was deleted conditionally in mice by using a hepatocyte-specific, adeno-associated virus-thyroxine binding globulin promoter-Cre recombinase viral construct. A marked increase in expression of PLAT (the tPA gene) was observed. This was accompanied by an increase in plasma tPA and evidence for activation of fibrinolysis in the vascular compartment. DACH1 seems to regulate tPA gene expression by repressing expression of ATF6, which is a major PLAT transcriptional inducer in hepatocytes. Knockdown of ATF6 in the livers of mice decreased plasma tPA protein and activity.
These investigators continued their work by demonstrating a major role for hepatocytes in contributing to the basal level of circulating tPA in wild-type mice, independently of DACH1 or ATF6 regulation. They provide a model to show how hepatocytes and vascular endothelium may contribute to the overall level of tPA in the plasma (see figure).
A strength of the study by Zheng et al is the translational work with deidentified human liver specimens. By using these specimens, the investigators demonstrated a correlation between ATF6 messenger RNA (mRNA) levels and tPA mRNA levels, which are both apparently controlled downstream of DACH1. The Zheng et al article offers a new vision for the therapeutic control of hemostasis. ATF6, DACH1, and other gene products that function in the same pathway to regulate hepatocyte tPA expression may be excellent targets for controlling the balance between coagulation and fibrinolysis. Controlling the basal level of tPA in patients may potentiate the antithrombotic response of injured blood vessels and vessels in which there is stasis or turbulent flow. Furthermore, recent studies implicating tPA in processes such as inflammation justify new studies that explore how regulation of basal levels of tPA affect activities that result from receptor interactions locally at the cellular level.5-8
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal