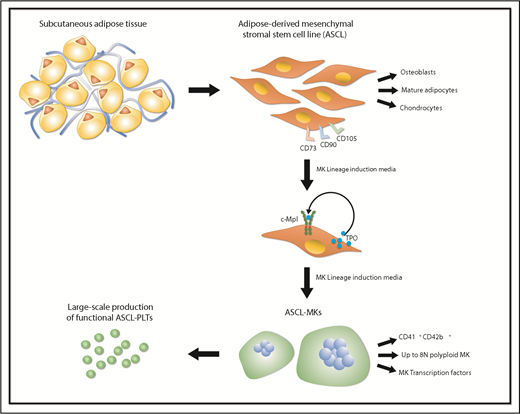
In this issue of Blood, Tozawa et al report the production of platelets from human adipose–derived mesenchymal stem/stromal cells for use in transfusion medicine as donor-independent source (see figure).1
Manufacturing platelets from human adipose–derived mesenchymal stem/stromal cells. Human subcutaneous adipose tissue was used to generate adipose-derived mesenchymal stromal/stem cell line (ASCL) that fully satisfies the criteria for defining mesenchymal stem cells as determined by The International Society for Cellular Therapy. When cultured in Megakaryocyte (MK) Lineage Induction Media, ASCL produces and secretes thrombopoietin (TPO), which binds to c-Mpl, supporting MK differentiation. Once mature, MKs can be cultured within a cell expansion system and generate functional human ASCL-Platelets (PLTs).
Manufacturing platelets from human adipose–derived mesenchymal stem/stromal cells. Human subcutaneous adipose tissue was used to generate adipose-derived mesenchymal stromal/stem cell line (ASCL) that fully satisfies the criteria for defining mesenchymal stem cells as determined by The International Society for Cellular Therapy. When cultured in Megakaryocyte (MK) Lineage Induction Media, ASCL produces and secretes thrombopoietin (TPO), which binds to c-Mpl, supporting MK differentiation. Once mature, MKs can be cultured within a cell expansion system and generate functional human ASCL-Platelets (PLTs).
Circulating platelets are highly specialized cells produced by megakaryocytes that participate in hemostatic functions. Despite their critical role, very little is known about the mechanisms regulating their production from megakaryocytes and even less is known about therapeutic approaches to modulate their release. Thrombocytopenia often requires platelet transfusions for patient treatment; however, the supply is limited and is entirely dependent on allogeneic donations. In addition, platelet transfusions expose patients to risks of disease transmission and induce acute reactions and alloimmunization, making the patients resistant to further infusions. Thus, scientific and clinical communities are actively searching for new ways of generating functional platelets ex vivo for clinical use and mechanistic studies.
Since the first description of an in vitro culture for the generation of human platelets and the cloning of human thrombopoietin 20 years ago, significant effort has been spent in developing different systems for successful megakaryocyte differentiation starting from hematopoietic stem cells derived from umbilical cord blood, adult peripheral blood, or bone marrow.2 All 3 cell sources have been used to provide insights into the basic mechanisms of human platelet production. However, the need for cells to overcome the limits of donor-derived platelet supply has also prompted several groups to develop efficient culture systems from human pluripotent stem cells, which can be divided into human embryonic stem cells and human-induced pluripotent stem cells (hiPSCs). hiPSCs are thought to be an inexhaustible source of megakaryocytes, able to produce human platelets duplicating peripheral blood in many aspects, including ultrastructure, surface antigen expression, and function. Nakamura et al developed an immortalized megakaryocyte progenitor cell line by the sequential introduction of c-MYC and BMI1 genes, followed by the BCL-XL gene into human hiPSC-derived hematopoietic progenitor cells,3 whereas Moreau et al established forward programmed megakaryocytes by overexpressing 3 transcription factors: GATA1, FLI1, and TAL1. These 3 transcription factors were introduced into human hiPSCs as part of a forward programming strategy to promote megakaryocyte differentiation under feeder-free conditions.4
Manufacturing clinically meaningful numbers of platelets is still difficult due to low platelet release from hiPSC-derived megakaryocytes. Recently, Ito et al identified turbulence as a physical regulator of platelet production from hiPSC-megakaryocytes and developed a turbulence-controlled bioreactor to scale up platelet production to a sufficient level for clinical applications.5 These data demonstrate that future advancement in the field will depend on the evolution of bioengineering techniques for reproducing physiologically relevant conditions to promote platelet formation ex vivo.6-9
Despite these important advancements, it is still unknown whether platelet production from megakaryocytes, derived from different progenitor sources, is regulated by alternative mechanisms. In other words, we still do not understand the regulation of platelet production. Until this is understood, it will be difficult to determine exact, safe triggers to promote a constant rate of platelet production. In addition, this uncertainty makes it difficult to determine the safety of the platelet products and the possible costs of the production process scale-up.
In searching for new strategies to produce platelets in a simple and cost-effective way, the group of Matsubara explored the use of adipose tissue–derived stromal cells (ASCs) as a source of megakaryocytes to produce platelets for clinical applications.10 First, they demonstrated that megakaryocytes could be differentiated from ASCs without the need of any genetic manipulation. Next, they showed that endogenous thrombopoietin supported megakaryocyte differentiation from ASCs. Finally, they proved that the endogenous thrombopoietin was secreted in a transferrin-dependent manner as blockage of the transferrin receptor CD-71 on ASCs caused a decrease of the thrombopoietin levels and megakaryocyte differentiation. The limit of the technique presented was that the ASCs were too heterogeneous to be used as a source of donor-independent megakaryocytes and platelets. Based on these studies, Tozawa et al developed an ASC cell line (ASCL) that was able to proliferate for 2 months without any alteration in the karyotype. In a selected culture medium, ASCL differentiated into megakaryocytes after 8 days, and platelets were released after an additional 4 days. Platelets produced ex vivo resembled peripheral blood platelets and showed the same in vivo kinetics after infusion into irradiated immunodeficient NSG mice. However, these platelets had a tendency to be hyperactive in vitro as demonstrated by higher levels of PAC1 binding, P-selectin surface exposure, and ristocetin-induced and ADP-induced platelet aggregation, although they showed similar levels of fibrinogen binding, collagen-induced platelet aggregation, and lower epinephrine-induced platelet aggregation compared with peripheral blood platelets. Although encouraging, these data raise the question on how to define platelets produced ex vivo that can be considered safe to be transfused from both a morphologic and a functional point of view. Further studies are needed to assess the cause of this increased response to platelet agonists and whether this preactivated state could impact the possibility of safely transfusing them in patients.
Finally, an intriguing point is that only 63% of ASCL differentiated into megakaryocytes. A possible explanation is that there are megakaryocyte-primed ASCLs among the ASCL population, and current studies are focused on understanding the role of mesenchymal markers in driving ASCL differentiation into megakaryocytes. These studies will be instrumental in understanding platelet production efficiency from these megakaryocytes and regulation of megakaryocyte maturation.
The study of Tozawa et al demonstrates a simple and inexpensive method to produce platelets from adipose tissue without the need for genetic manipulation and exogenous growth factors. In conclusion, substantial advances have been made in the field; however, bona fide generation of blood cells and platelets is still an unmet need requiring a definition of functional progenitors, megakaryocytes, and platelets.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal