In this issue of Blood, Baker et al investigated the role of total body irradiation (TBI) dose and fractionation on the risk of subsequent malignant neoplasms (SMNs) after allogeneic hematopoietic cell transplantation (HCT). Their findings suggest a strong effect of both on the risk of SMNs after HCT.1
Over the past 2 decades, HCT activity has steadily increased worldwide, thanks to the availability of more donors, lower toxicity, and improved supportive care.2 Therefore, immediate survival is no longer the sole concern after HCT. Complete recovery of health, including active physical and psychological functioning, normal family and social integration, and the subjective sense of well-being, are of paramount importance. Ablative doses of TBI plus chemotherapy used prior to HCT have usually been associated with a high incidence of SMNs after HCT. This may be linked to several factors, such as (1) the mutagenic effects of irradiation and chemotherapy, (2) genetic predisposition and age-related factors, and (3) prolonged immunosuppression.3 In a cohort of 1036 patients who received HCT before December 1985, and who survived more than 5 years, the actuarial incidence of SMNs was 3.5% at 10 years and 12.8% at 15 years. This is 3.8-fold higher than that in an age-matched control population.4 In general, secondary leukemias occur earlier than solid tumors (melanoma, liver, brain, bone, breast, connective tissue, squamous-cell cancers of the buccal cavity, and skin).5 In contrast to high-dose TBI-based conditioning, the use of chemotherapy-based conditioning regimens before HCT is correlated with a lower incidence of SMNs. In a study of 4318 patients who received high-dose busulfan and cyclophosphamide conditioning, the risk of solid cancers at 10 years after HCT was 1.4 times higher than the expected rate.6
In the late 1990s, the development of reduced-intensity or nonmyeloablative conditioning regimens, especially those based on low-dose TBI, represented a major breakthrough in the field of HCT. Such regimens reduced the immediate toxicity and mortality after HCT, and the characteristics of late HCT-related complications were significantly modified after such reduced regimens.7
On the basis of this background, Baker et al hypothesized that the use of these low-intensity conditioning regimens would impact post-HCT development of SMNs. Thus, they investigated the SMN incidence in a cohort of 4905 patients who received various intensity conditioning regimens prior to HCT. They found that low-dose TBI (defined arbitrarily as a dose of 200 to 450 cGy), is still correlated with a relatively high risk of SMNs (twofold higher than in the general population), although this was lower than the incidence observed after fractionated or unfractionated high-dose TBI-based conditioning.
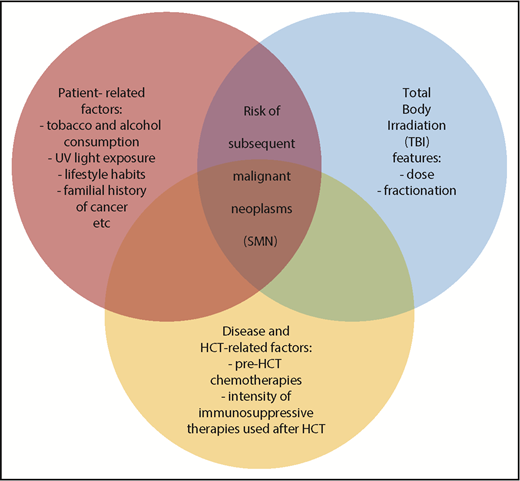
There are some limitations to this analysis, such as its retrospective nature, a possible under reporting of SMN cases, and the relatively shorter follow-up in patients who received low-dose TBI. In addition, the definition of low-dose TBI was based on the classical clinical judgment, which may not accurately reflect the level of tissue injury leading to SMN development. Moreover, important risk factors such as tobacco and alcohol consumption, UV light exposure, lifestyle habits, familial history of cancer, pre-HCT chemotherapy, or the intensity of immunosuppressive therapy after HCT were not captured. All the latter parameters can significantly influence the overall risk of SMN development after HCT.
In spite of these limitations, the results of Baker et al are revealing and can pave the way for further research and intervention in this field, because SMN occurrence after HCT is a catastrophic complication. Indeed, low-dose TBI is now widely used for conditioning in elderly patients, and in young patients with comorbidities that preclude the use of TBI or high-dose chemotherapy. Although low-dose TBI can significantly reduce the incidence of short-term complications, the development of SMNs is still a matter of concern. Furthermore, it is likely that the timing of SMN development will change in the future, given the rapidly increasing age of HCT candidates. The current study by Baker et al, sheds further light on the fact that long-term adverse effects after HCT, including SMNs, are a multifactorial and complex process (see figure). Reducing the rate of short term toxicity as a consequence of a milder conditioning is not sufficient to abrogate some serious and life-threatening long-term complications, and for many patients, long-term survival is not accompanied by full restoration of health.
Life-long monitoring, counseling, and prevention and treatment are mandatory through a close partnership between the transplant center, organ-specific specialties, and local primary care providers.8 The human papillomavirus (HPV) vaccine is one example of a possible effective intervention to prevent HPV-related diseases, including cervical, vulvar, and vaginal cancers and precancers in females, as well as anal cancers, precancers, and genital warts in both females and males, regardless of previous sexual activity or exposure to carcinogenic strains.9 The patient can also play a major role through compliance with health maintenance behaviors.
The optimal care model post-HCT over the long term is yet to be defined. When this happens, it must also be adapted to local constraints. The report by Baker et al is a significant step in the right direction to achieving these goals, because we must be prepared to deal with the consequences of our own therapeutic successes.
Conflict-of-interest disclosure: The author declares no competing financial interests.