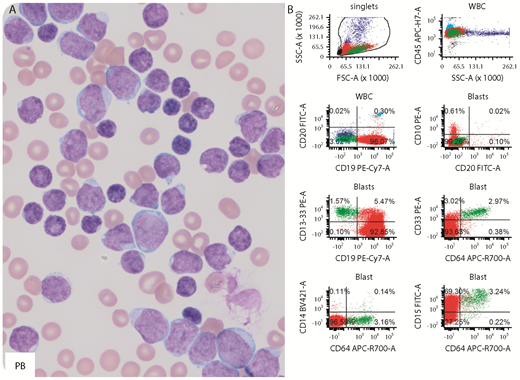
A 12-year-old girl had marked leukocytosis (white blood cell count, 692 000/µL), severe anemia (hemoglobin, 5.9 g/dL), and thrombocytopenia (platelet count, 13 000/µL). Peripheral blood (PB) and bone marrow (BM) smears appeared similar with numerous small to large blasts (panel A; Wright-Giemsa stain, original magnification ×1000). Flow cytometry detected 2 distinct populations: B lymphoblasts (PB, 93%; BM, 96%) and monoblasts (PB, 2.0%; BM, 3.0%). The lymphoblasts were positive for CD19, partial CD34, and CD15 and negative for CD10 and CD20 (panel B, red), a pattern frequently seen in B-cell acute lymphoblastic leukemia (ALL) with t(4;11) KMT2A-AFF1–rearranged. The monoblasts were intermediate to large, expressed heterogeneous CD11C, bright CD15, CD33, and CD64 and were negative for CD19, CD34, CD13, CD14, and CD117 (panel B, green). The patient was diagnosed as mixed-phenotype acute leukemia with t(4;11) KMT2A-AFF1–rearranged upon confirmation by karyotyping and fluorescence in situ hybridization (FISH) (91.3% in BM). Residual disease was detected by flow cytometry on day 29 in BM after ALL induction therapy (0.3% B lymphoblasts and 3.8% monoblasts) and on day 29 in BM after ALL consolidation therapy (0.17% B lymphoblasts and 4.7% monoblasts). FISH analysis detected KMT2A rearrangement in 4.8% cells in the BM after consolidation.
This case highlighted the power of flow cytometry in diagnosing leukemia. It illustrates the important point that small but significant abnormal populations can be obscured by large ones.
A 12-year-old girl had marked leukocytosis (white blood cell count, 692 000/µL), severe anemia (hemoglobin, 5.9 g/dL), and thrombocytopenia (platelet count, 13 000/µL). Peripheral blood (PB) and bone marrow (BM) smears appeared similar with numerous small to large blasts (panel A; Wright-Giemsa stain, original magnification ×1000). Flow cytometry detected 2 distinct populations: B lymphoblasts (PB, 93%; BM, 96%) and monoblasts (PB, 2.0%; BM, 3.0%). The lymphoblasts were positive for CD19, partial CD34, and CD15 and negative for CD10 and CD20 (panel B, red), a pattern frequently seen in B-cell acute lymphoblastic leukemia (ALL) with t(4;11) KMT2A-AFF1–rearranged. The monoblasts were intermediate to large, expressed heterogeneous CD11C, bright CD15, CD33, and CD64 and were negative for CD19, CD34, CD13, CD14, and CD117 (panel B, green). The patient was diagnosed as mixed-phenotype acute leukemia with t(4;11) KMT2A-AFF1–rearranged upon confirmation by karyotyping and fluorescence in situ hybridization (FISH) (91.3% in BM). Residual disease was detected by flow cytometry on day 29 in BM after ALL induction therapy (0.3% B lymphoblasts and 3.8% monoblasts) and on day 29 in BM after ALL consolidation therapy (0.17% B lymphoblasts and 4.7% monoblasts). FISH analysis detected KMT2A rearrangement in 4.8% cells in the BM after consolidation.
This case highlighted the power of flow cytometry in diagnosing leukemia. It illustrates the important point that small but significant abnormal populations can be obscured by large ones.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal