TO THE EDITOR:
The receptor for interleukin-7 (IL-7) is formed by the IL7RA and the IL2RG (γc) chains. IL-7 leads to the reciprocal phosphorylation of IL7RA-associated JAK1 and γc-associated JAK3, followed by phosphorylation of IL7RA and then STAT5 anchoring and activation. Constitutive signaling mutants of IL7RA were described in acute lymphoblastic leukemia (ALL). So far, the mutations studied include extracellular juxtamembrane (EJM) cysteine insertions1-3 and transmembrane (TM) cysteine that lacks insertions,4 both of which enable homodimer formation and constitutive signaling independently of cytokine and γc. In this study, we describe another class of IL7RA mutation characterized by the insertion of positively charged amino acids in the EJM region of the receptor. In contrast to the cysteine EJM and TM insertion mutations, the EJM charged residue mutations are able to activate signaling and confer proliferative advantage only in the presence of both IL-7 and γc but with greatly increased sensitivity to IL-7.
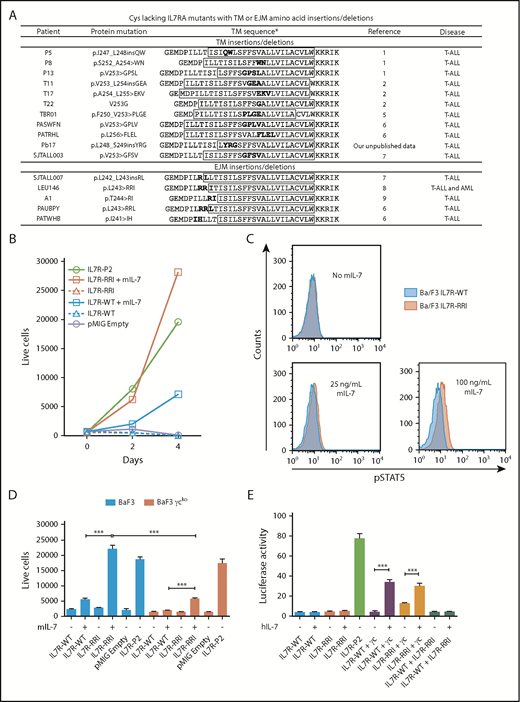
Figure 1A presents all previously published cysteine-lacking IL7RA mutations separated into 2 different groups: insertions/deletions deep in the TM region and insertions/deletions in the EJM region.1,2,4-9 Interestingly, the mutations in the EJM region have positively charged amino acids such as arginine (R) and histidine (H). The IL7RA mutation p.L243>RRI (IL7R-RRI) was chosen for investigation.
Oncogenic potential of IL7R-RRI is IL-7– and γc-dependent. (A) TM and EJM cysteine–lacking mutations found in the literature. *TM regions predicted by TMPred (http://www.ch.embnet.org/software/TMPRED_form.html) are shown inside the boxes. (B) Ba/F3 cells stably expressing IL7R-WT, IL7R-RRI, IL7R-P2, or the empty vector (pMIG) cultured with or without mouse IL-7 (mIL-7; 5 ng/mL). The number of viable GFP+ cells per 1000 PercP-labeled beads (BD, Franklin Lakes, NJ) was determined by flow cytometry. (C) Ba/F3 cells stably expressing IL7R-WT or IL7R-RRI were starved for 4 hours (without fetal bovine serum, IL-3, or IL-7), stimulated with different concentrations of mIL-7 over 15 minutes, and used to detect phosphorylated STAT5 (pSTAT5) by intracellular flow cytometry. (D) Ba/F3 cells (blue bars) or γc-knockout Ba/F3 cells (red bars) were transduced with pMIG empty vector, IL7R-WT, IL7R-RRI, or IL7R-P2 and cultured with or without mIL-7 (5 ng/mL). The number of viable GFP+ cells per 1000 beads was determined by flow cytometry after 48 hours. (E) HEK293T cells were transiently transfected with different combinations of IL7RA and γc constructs in conjunction with JAK3 and Renilla luciferase expression vectors plus the STAT3/5-responsive luciferase reporter construct. Medium was replaced 16 hours after transfection. Eight hours later, IL-7 or vehicle was added, and the cells were cultured for 12 hours before luciferase was measured in a plate reader. Firefly luciferase was normalized by Renilla levels. Data are representative of at least 2 independent experiments. Results are the mean ± standard deviation (SD) of triplicate samples. Data were analyzed by using analysis of variance (ANOVA) and Tukey post hoc test. ***P < .001.
Oncogenic potential of IL7R-RRI is IL-7– and γc-dependent. (A) TM and EJM cysteine–lacking mutations found in the literature. *TM regions predicted by TMPred (http://www.ch.embnet.org/software/TMPRED_form.html) are shown inside the boxes. (B) Ba/F3 cells stably expressing IL7R-WT, IL7R-RRI, IL7R-P2, or the empty vector (pMIG) cultured with or without mouse IL-7 (mIL-7; 5 ng/mL). The number of viable GFP+ cells per 1000 PercP-labeled beads (BD, Franklin Lakes, NJ) was determined by flow cytometry. (C) Ba/F3 cells stably expressing IL7R-WT or IL7R-RRI were starved for 4 hours (without fetal bovine serum, IL-3, or IL-7), stimulated with different concentrations of mIL-7 over 15 minutes, and used to detect phosphorylated STAT5 (pSTAT5) by intracellular flow cytometry. (D) Ba/F3 cells (blue bars) or γc-knockout Ba/F3 cells (red bars) were transduced with pMIG empty vector, IL7R-WT, IL7R-RRI, or IL7R-P2 and cultured with or without mIL-7 (5 ng/mL). The number of viable GFP+ cells per 1000 beads was determined by flow cytometry after 48 hours. (E) HEK293T cells were transiently transfected with different combinations of IL7RA and γc constructs in conjunction with JAK3 and Renilla luciferase expression vectors plus the STAT3/5-responsive luciferase reporter construct. Medium was replaced 16 hours after transfection. Eight hours later, IL-7 or vehicle was added, and the cells were cultured for 12 hours before luciferase was measured in a plate reader. Firefly luciferase was normalized by Renilla levels. Data are representative of at least 2 independent experiments. Results are the mean ± standard deviation (SD) of triplicate samples. Data were analyzed by using analysis of variance (ANOVA) and Tukey post hoc test. ***P < .001.
The wild-type (WT) and mutant human IL7RA were cloned into the pMIG (MSCV-IRES-EGFP) retroviral vector and transduced into the IL-3–dependent Ba/F3 cell line. Green fluorescent protein–positive (GFP+) cells were sorted by flow cytometry (a detailed description of the methods is provided in the supplemental Data available on the Blood Web site). In the absence of IL-3 and IL-7, Ba/F3 cells transduced with the IL7R-RRI or IL7R-WT died, whereas those transduced with the IL7RA cysteine–mutant IL7R-P21 proliferated (Figure 1B). However, in the presence of IL-7 (Peprotech, Rocky Hill, NJ), Ba/F3 cells expressing IL7R-RRI proliferated more and showed higher levels of phosphorylated STAT5 (p-STAT5) (Figure 1C; supplemental Figure 1) than cells expressing IL7R-WT. Cells expressing IL7R-P2 did not respond to IL-7 (supplemental Figure 2).
Ba/F3 cells express the mouse IL2RG (supplemental Figure 3). To investigate whether the IL7R-RRI acts as a heterodimer with γc or as a homodimer, we mutated the IL2RG gene in Ba/F3 cells in a significant proportion of cells using the CRISPR/Cas9 system (supplemental Figure 4) and transduced the resulting pool (not a cell line) of cells (Ba/F3-γcko) with the IL7R constructs. The IL-7–induced survival/proliferation of cells expressing the IL7R-RRI receptor was almost abrogated in Ba/F3-γcko cells (Figure 1D). Cells transduced with the positive control cysteine-type IL7R-P2 mutant grew equally well, irrespective of γc mutation. To confirm this finding, HEK293T cells were transfected with homodimeric or heterodimeric combinations of IL7R-WT, IL7R-RRI, and the human γc in conjunction with a JAK3 expression vector and a STAT3/5 luciferase reporter vector. Twenty-four hours after transfection, cells were cultured for an additional 12 hours with different IL-7 concentrations. Luciferase was measured (Dual-Luciferase Assay, Promega, Madison, WI), and Firefly activity was normalized by Renilla luciferase. As shown in Figure 1E, the co-expression of the human γc was essential for IL-7 activation of the IL7R-RRI receptor.
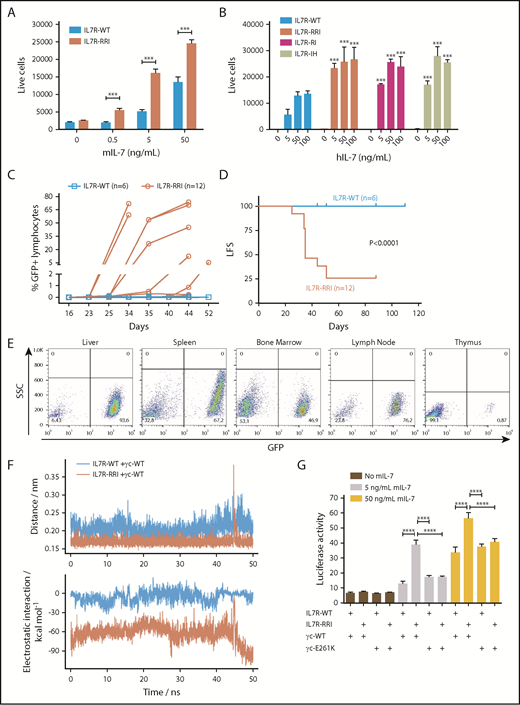
Cysteine-type EJM and cysteine-free TM IL7RA mutations both result in constitutive receptor activation, which is a significant competitive advantage for the cell in view of the limiting amount of IL-7.10 We found that the IL7R-RRI provides a growth advantage over the IL7R-WT receptor at lower IL-7 concentrations. IL7R-RRI–transduced cells were approximately 10 times more sensitive to IL-7 than IL7R-WT cells were; the proliferation of IL7R-RRI cells at 0.5 and 5 ng/mL was similar to that of IL7R-WT cells at 5 and 50 ng/mL, respectively (Figure 2A; supplemental Figure 5). Compatible results were obtained with 2 other IL7R mutants (Figure 2B) and in the luciferase reporter assay (supplemental Figure 6). Of note, the cell surface expression of IL7R-RRI and IL7R-WT was similar (supplemental Figure 3), and the amounts of IL-7 used in these experiments were comparable to normal IL-7 levels in the mouse bone marrow (∼30 ng/mL).11 Importantly, IL-7 hypersensitivity was seen in T-cell ALL (T-ALL)12 and in connection with glucocorticoid resistance.13 Our work describes a potential mechanism by which T-ALL could become hypersensitive to IL-7.
In vivo leukemogenesis and increased response to IL-7 as a result of putative electrostatic interactions between the EJM regions of IL7R-RRI and γc. (A) Ba/F3 ectopically expressing IL7R-WT or IL7R-RRI were cultured with different concentrations of mIL-7, and cell viability (GFP+ cells per 1000 beads) was evaluated after 48 hours by flow cytometry. (B) Unsorted Ba/F3 cells ectopically expressing IL7R-WT, IL7R-RRI, IL7R-RI (p.T244>RI), or IL7R-IH (p.I241>IH) were cultured with different concentrations of human IL-7 (hIL-7), and numbers of GFP+ cells per 1000 beads were evaluated after 96 hours by flow cytometry. (C) Percentage of GFP+ cells in peripheral blood lymphocytes and (D) Kaplan-Meier curves (GFP+ cells >0.1% in peripheral blood lymphocytes being the event) of C3H/HePas isogenic mice injected with Ba/F3 cells expressing IL7R-WT or IL7R-RRI. Animals injected with IL7R-WT Ba/F3 cells were observed for 110 days with no sign of engraftment. The IL7R-P2 was not tested in C3H/HePas mice, but in Balb/c and NOD/SCID mice, engraftment occured in about 2 weeks. Curves in (D) were compared by the log-rank test. (E) Infiltration of GFP+ cells in different organs of representative mice injected with IL7R-RRI Ba/F3 cells. SSC, side scatter. (F) Molecular dynamics simulations of the IL7RA and the γc transmembrane peptides interacting in a lipid bilayer. ns, nanoseconds. Peptide associations were stable (upper panel). In the wild-type pair the electrostatic interactions were characteristic of nonpolar bonds. Much stronger electrostatic interactions were observed for the IL7R-RRI/γc pair (lower panel) as a result of bonds between arginine (R243) and γc E261 of IL7R-RRI (supplemental Figure 8). (G) STAT3/5-luciferase transactivation assay performed in HEK293T cells as above, using different combinations of IL7RA (in pMIG) and γc constructs (in pcDNA3.1+-C-DYK). Data are representative of at least 2 independent experiments. Results are the mean ± SD of triplicate samples. Data were analyzed by ANOVA and Tukey post hoc test. ***P < .001; ****P < .0001.
In vivo leukemogenesis and increased response to IL-7 as a result of putative electrostatic interactions between the EJM regions of IL7R-RRI and γc. (A) Ba/F3 ectopically expressing IL7R-WT or IL7R-RRI were cultured with different concentrations of mIL-7, and cell viability (GFP+ cells per 1000 beads) was evaluated after 48 hours by flow cytometry. (B) Unsorted Ba/F3 cells ectopically expressing IL7R-WT, IL7R-RRI, IL7R-RI (p.T244>RI), or IL7R-IH (p.I241>IH) were cultured with different concentrations of human IL-7 (hIL-7), and numbers of GFP+ cells per 1000 beads were evaluated after 96 hours by flow cytometry. (C) Percentage of GFP+ cells in peripheral blood lymphocytes and (D) Kaplan-Meier curves (GFP+ cells >0.1% in peripheral blood lymphocytes being the event) of C3H/HePas isogenic mice injected with Ba/F3 cells expressing IL7R-WT or IL7R-RRI. Animals injected with IL7R-WT Ba/F3 cells were observed for 110 days with no sign of engraftment. The IL7R-P2 was not tested in C3H/HePas mice, but in Balb/c and NOD/SCID mice, engraftment occured in about 2 weeks. Curves in (D) were compared by the log-rank test. (E) Infiltration of GFP+ cells in different organs of representative mice injected with IL7R-RRI Ba/F3 cells. SSC, side scatter. (F) Molecular dynamics simulations of the IL7RA and the γc transmembrane peptides interacting in a lipid bilayer. ns, nanoseconds. Peptide associations were stable (upper panel). In the wild-type pair the electrostatic interactions were characteristic of nonpolar bonds. Much stronger electrostatic interactions were observed for the IL7R-RRI/γc pair (lower panel) as a result of bonds between arginine (R243) and γc E261 of IL7R-RRI (supplemental Figure 8). (G) STAT3/5-luciferase transactivation assay performed in HEK293T cells as above, using different combinations of IL7RA (in pMIG) and γc constructs (in pcDNA3.1+-C-DYK). Data are representative of at least 2 independent experiments. Results are the mean ± SD of triplicate samples. Data were analyzed by ANOVA and Tukey post hoc test. ***P < .001; ****P < .0001.
The competitive advantage conferred by the IL7R-RRI mutation could be also demonstrated in vivo under physiological IL-7 levels and the interaction and competition with other cells. The study was approved and registered by the Animal Ethics Committee from the State University of Campinas (#4770-1/2017). One million Ba/F3 cells transduced with the different IL7R constructs were injected intravenously into syngenic female 6- to 8-week-old C3H/HePas mice, and the presence of GFP+ cells was monitored by flow cytometry. Mice injected with IL7R-RRI–expressing Ba/F3 cells started developing leukemia by week 4 (Figure 2C-D). The leukemogenic effect of IL7R-RRI mutation was also evidenced by the infiltration of Ba/F3 cells in the different organs (liver, spleen, bone marrow, lymph nodes, and thymus; Figure 2E). Conversely, mice injected with IL7R-WT cells were healthy and had no Ba/F3 cell infiltration until the end of the experiment at 110 days.
Charge interactions between EJM regions of homodimeric growth hormone receptor chains play an important role in receptor activation.14-16 Molecular dynamics simulations suggested that the electrostatic interactions between the IL7R-RRI mutant and the γc are much more favorable than those of the WT IL7R because of direct R244-E261 interactions (Figure 2F; supplemental Figures 7 and 8). To confirm this possibility, we performed STAT3/5 luciferase reporter assays with a mutant human γc receptor in which the acidic EJM glutamic acid residue (E261) was replaced by the positively charged residue lysine (supplemental Figure 9). As shown in Figure 2G, this single amino acid substitution in the EJM domain of the γc resulted in a significant loss of IL7R-RRI/γc signaling to levels as low as those seen for the IL7R-WT/γc heterodimer. Mutations at more distant charged residues (K252E and E253K) in the γc had no effect (supplemental Figure 10). Moreover, the EJM of IL7RA has two acidic residues (E238 and D240). As expected, an acidic-to-basic mutation (E238K/D240R) resulted in IL-7 hypersensitivity, whereas no effect was seen when these residues were mutated to neutral alanine (supplemental Figure 11).
How is it that positively charged EJM (+EJM) IL7RA mutations confer increased sensitivity for IL-7? The +EJM mutations are too distant from the elbow loop residues that act on IL-7 binding,17 and thus are not likely to have an influence on IL7RA affinity for the IL-7 ligand. We were able to show that the positively charged residues in the EJM of IL7RA may align and electrostatically interact with a negatively charged residue in the EJM of the γc chain. Mutagenesis of this single negatively charged residue in the EJM of the γc chain receptor abrogated the surplus signaling by the IL7R-RRI/γc heterodimer. Enhanced approximation between the EJM regions of the IL7R and γc chains may lead to conformational changes necessary for JAK activation14 and to increased cell surface preassembly of the IL7R-RRI/γc heterodimer. Importantly, by showing productive electrostatic interaction between the IL7RA and γc chains, our data strongly suggest that the N-terminal portions of the transmembrane domains of IL7RA and γc come into close proximity upon IL-7 activation, much closer than the 30 Å previously suggested.18
In conclusion, this work describes a new class of IL7RA mutations characterized by the presence of charged amino acids in the EJM domain, which create an electrostatic motif that facilitates interaction with the γc and enhances signaling upon IL-7 activation.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Laurent Knoops (Université Catholique de Louvain, Louvain-la-Neuve, Belgium), Thierry Rose (Institut Pasteur, Paris, France), and Isabelle Lucet (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for kindly providing the pLHRE-luc plasmid, human γc clone, and the human JAK3 clone, respectively.
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (12/12802-1 and 14/20015-5) (J.A.Y.) and (10/16947-9 and 13/08293-7) (L.M.), and by a grant from the National Health and Medical Research Council of Australia (APP1084797) (A.J.B.). L.W.C., G.O.L.R., and L.L.A. received scholarships from Fundação de Amparo à Pesquisa do Estado de São Paulo. L.G.P. received a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. J.A.Y. received a Productivity Fellowship from the Brazilian National Counsel of Technological and Scientific Development (CNPq 305896/2013-0 and 301596/2017-4).
Authorship
Contribution: L.W.C., L.F.A., and J.A.Y. conceived and designed the study; L.W.C. performed all experiments; L.G.P. created the Ba/F3 cell line with γc knockout; G.O.L.R. performed transduction of Ba/F3 cells with the different IL7R constructs; P.P.Z. and T.R.G. performed in vivo experiments and fluorescence-activated cell sorting analysis of IL7R and γc expression; L.L.A. performed Ba/F3 proliferation assays and helped with the luciferase assays; L.M. performed molecular dynamics simulations; L.W.C., P.P.Z., L.M., A.J.B., and J.A.Y. analyzed results; and L.W.C., P.P.Z., L.M., A.J.B., and J.A.Y. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: José Andrés Yunes, Centro Infantil Boldrini, Rua Dr Gabriel Porto 1270, Campinas, SP 13083-210, Brazil; e-mail: andres@boldrini.org.br.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal