Abstract
Introduction
Cellular therapy with chimeric antigen receptor (CAR) T cells has fundamentally changed the treatment of many cancers. Unfortunately, not all patients who receive this therapy have a favorable response. Additionally, some patients may develop toxicity due to cytokine release syndrome (CRS) or neurotoxicity. Recent studies have found a relationship between the intestinal microbiome and the response to immunotherapy with checkpoint blockade. We propose the intestinal microbiota as a factor that influences the efficacy and toxicity of CAR T cells. We hypothesize that the differences in outcomes of patients who receive CAR T cells are related to the composition of their intestinal microbiota at baseline. We report a single-center analysis of pre-CAR T cell infusion microbiota composition.
Methods
We collected stool samples from recipients of CAR T cells at Memorial Sloan Kettering Cancer Center (MSKCC). A baseline sample was collected prior to CAR T cell infusion. Samples were submitted for 16S RNA sequencing of the V4-V5 region on the Illumina MiSeq platform and the operational taxonomic units (OTUs) were classified using the NCBI Reference Sequence Database. Clinical response to assess efficacy was classified as either complete response (CR) or no complete response. Given the sample size, toxicity was pooled to encompass Grade 1 to 4 CRS and Grade 1 to 4 neurotoxicity. Linear discriminant analysis effect size (LEfSe) was used to identify microbial biomarkers for efficacy and toxicity between groups using relative abundances with a linear discriminant analysis score threshold >2.5.
Results
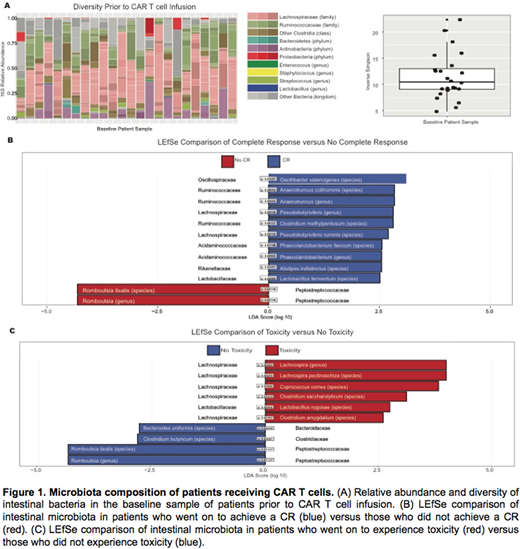
We analyzed 24 baseline samples from 24 patients treated at MSKCC. The patients were all adult recipients of cellular therapy with CAR T cells. The patients varied in conditioning regimen, CAR construct and underlying diagnosis, which included solid tumors and hematologic malignancies. First, we assessed the 16S relative abundance of the intestinal microbiota of the patients at baseline. We found that the composition of the microbiota prior to CAR T cell infusion was diverse, as defined by an Inverse Simpson >4 in all of the patients, although the level of diversity amongst the patient samples varied (Fig A). An assessment of the efficacy of CAR T cells with LEfSe analysis found increased abundance in several families of the Clostridiales order (Firmicutes phylum), including Oscillospiraceae, Ruminococcacaeae, and Lachnospiraceae, in those patients who achieved a CR. For the patients who did not achieve a CR, we found an increased abundance of a family in the Clostridiales order (Firmicutes phylum), Peptostreptococcaceae. Patients who experienced toxicity, either CRS or neurotoxicity, had an increased abundance of families within the Clostridiales or Lactobacillales order (Firmicutes phylum), which included Lachnospiraceae and Lactobacillaceae. Finally, patients who did not experience toxicity also had an increased abundance of a family in the Clostridiales order (Firmicutes phylum), Peptostreptococcaceae.
Conclusion
We demonstrate that our subset of patients had diverse microbial composition prior to receiving CAR T cell therapy despite the fact that many of them were heavily pre-treated. Additionally, we observe the abundance of the family Lachnospiraceae in the patients who achieved a CR and those who experienced toxicity. Many Lachnospiraceae are butyrate producers, whose presence has been found to be protective against Clostridium difficile infection in recipients of allogeneic hematopoietic cell transplant but whose abundance is lower in colon cancer. Conversely, we observe an abundance of the family Peptostreptococcaceae in patients who did not achieve a CR or who did experience toxicity. Peptostreptococcaceae has been found to be more abundant in the intestines of patients with colon cancer. Of note, the intestinal micriobiota that we identify are not congruent with the specific bacteria that have been found to promote anti-tumor immunity to checkpoint blockade. Our data suggests a role for the intestinal microbiota in mediating the response to CAR T cells and proposes that the baseline microbial composition may correlate with efficacy and toxicity. Further studies will investigate biochemical mechanisms to understand the interplay of the intestinal microbiota and the immune system to improve patient outcomes following CAR T cell therapy.
Park:Adaptive Biotechnologies: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Novartis: Consultancy; AstraZeneca: Consultancy; Kite Pharma: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Shire: Consultancy. O'Cearbhaill:Juno: Research Funding. Mailankody:Juno: Research Funding; Janssen: Research Funding; Takeda: Research Funding; Physician Education Resource: Honoraria. Smith:Celgene: Consultancy, Patents & Royalties: CAR T cell therapies for MM, Research Funding. Palomba:Pharmacyclics: Consultancy; Celgene: Consultancy. Riviere:Fate Therapeutics Inc.: Research Funding; Juno Therapeutics, a Celgene Company: Membership on an entity's Board of Directors or advisory committees, Research Funding. Brentjens:Juno Therapeutics, a Celgene Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal