Abstract
Introduction: Denosumab is a monoclonal antibody targeting receptor activator of nuclear factor-kappa B ligand (RANKL) that has been shown to reduce skeletal-related events (SREs) associated with bone lesions in patients with multiple myeloma. Results from the full primary analysis of an international, double-blind, double-dummy, randomized controlled phase 3 (20090482) study that assessed the efficacy and safety of denosumab vs zoledronic acid for preventing SREs in patients with multiple myeloma (MM) indicated that denosumab was non-inferior to zoledronic acid for time to SREs. Here we present a sub-analysis to evaluate efficacy and safety outcomes in a subgroup of Asian patients enrolled in the 20090482 study.
Methods: Adult patients from Asian countries with newly diagnosed MM and ≥1 documented lytic bone lesion were included in this analysis. Patients received subcutaneous denosumab (120 mg) plus intravenous placebo or intravenous zoledronic acid (4 mg) plus subcutaneous placebo in 4-week cycles. The primary endpoint was time to first on-study SRE; the incidence of adverse events (AEs) by preferred term was also examined.
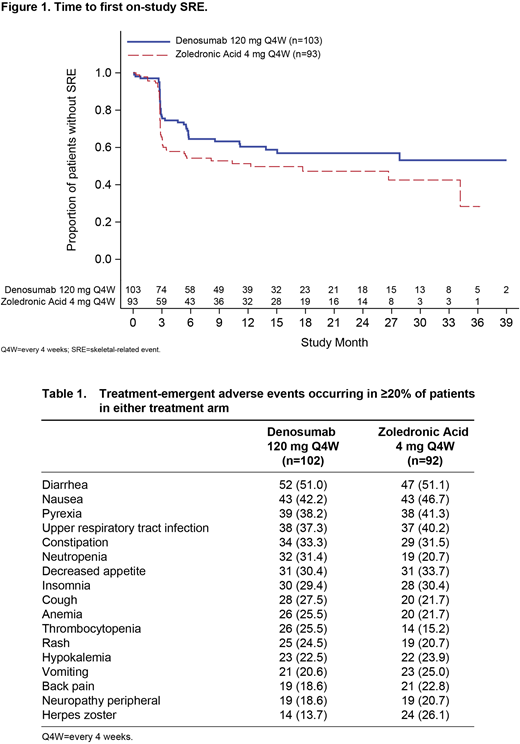
Results: Overall, 196 Asian patients (denosumab, n=103; zoledronic acid, n=93) were included in this analysis. Patient demographics were generally well balanced between groups. Median (interquartile range [IQR]) number of months on study was 17.5 (9.8-30.2) for the denosumab group and 20.2 (13.1-29.2) for the zoledronic acid group. Median (IQR) cumulative drug exposure was 15.9 (8.5-24.0) months for denosumab and 17.4 (9.1-26.7) months for zoledronic acid. Fewer patients in the denosumab group developed first on-study SRE compared with the zoledronic acid group; the crude incidence of SREs at the primary analysis cutoff was 38.8% in the denosumab group and 50.5% in the zoledronic acid group. Median (95% CI) time in months to first on-study SRE was not reached (11.2-not reached) for the denosumab group and 12.3 (3.1-not reached) for the zoledronic acid group (hazard ratio [HR], 0.77; 95% CI, 0.48-1.26; Figure 1). Overall, all patients (100%) experienced ≥1 treatment-emergent AE; the AEs reported in ≥20% of patients in either treatment arm are presented in Table 1. The most common AEs reported in either subgroup (denosumab, zoledronic acid) were diarrhea (51.0%, 51.1%), nausea (42.2%, 46.7%), pyrexia (38.2%, 41.3%), upper respiratory tract infection (37.3%, 40.2%), and constipation (33.3%, 31.5%). Renal toxicity (preferred terms of blood creatinine increased, renal failure, urine output decreased, acute kidney injury, renal impairment, and blood urea decreased) occurred in 9 of 102 (8.8%) patients in the denosumab group and 20 of 92 (21.7%) patients in the zoledronic acid group. Adjudicated osteonecrosis of the jaw was reported in 7 (6.9%) patients in the denosumab group and 5 (5.4%) patients in the zoledronic acid group. Hypocalcemia was reported in 19 (18.6%) patients in the denosumab group and 17 (18.5%) patients in the zoledronic acid group.
Conclusion: Results from this Asian subgroup analysis were comparable to those of the full analysis set. In addition, in this analysis there were numerically fewer patients in the denosumab arm that developed a first on-study SRE compared with those in the zoledronic acid arm, and the time to first on-study SRE had a trend favoring the denosumab-treated patients. The AE profiles for denosumab and zoledronic acid in the Asian subgroup were comparable to those observed in the full primary analysis, with renal toxicity similarly reported to be higher in the zoledronic acid group. Overall, this analysis supports that denosumab may be an additional treatment option for the standard of care for Asian patients with newly diagnosed MM with bone disease.
Chng:Merck: Research Funding; Aslan: Research Funding; Celgene: Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel, accommodation, expenses; Amgen: Consultancy, Honoraria, Other: Travel, accommodation, expenses. Chang:BMS: Consultancy, Speakers Bureau; AbbVie: Consultancy; Janssen: Consultancy, Speakers Bureau; Celgene: Consultancy; Takeda: Consultancy; Roche: Consultancy, Speakers Bureau; Novarits: Consultancy, Speakers Bureau. Wong:Amgen: Consultancy, Research Funding, Speakers Bureau; Bayer: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Archigen: Research Funding; Baxalta: Research Funding; Pfizer: Research Funding; Apellis: Research Funding; Roche: Research Funding; Boehringer: Research Funding; Ingelheim: Research Funding; AbbVie: Research Funding; Alexion: Consultancy; Astellas: Speakers Bureau. Shimizu:Amgen Inc.: Other: Non-remunerative Position of Influence, Denosumab 20090482 Global Steering Committee Member; Fujimoto Pharmacuetical Corp: Consultancy; Daiichi-Sankyo, Co., Ltd: Consultancy. Gao:Amgen Asia Holding Limited: Employment, Equity Ownership. Glennane:Amgen: Employment, Equity Ownership. Guan:Amgen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal