Abstract
Background: Polycythemia vera (PV) and essential thrombocythemia (ET) are myeloproliferative neoplasms (MPN) that can progress to post-PV (PPV) myelofibrosis (MF) and post-ET (PET) MF, from now on referred to as secondary myelofibrosis (SMF). Recent studies have shown an increased risk of developing solid tumors (ST) in MPN patients in comparison to the general population. Information on development of ST in SMF is scant.
Objectives of this study are to investigate ST in SMF correlating clinical phenotypes and treatments and to evaluate differences in the incidence of ST between PV and ET patients who developed SMF and those who did not.
Methods: The SMF group (including only PV and ET who developed SMF) was from the MYSEC cohort with ST-data collected (n=768 SMF); the PV/ET group including only patients who did not evolved into SMF at the time of this analysis was from the Pavia cohort (n=1452, 611 PV and 841 ET). SMF diagnosis was performed according to the IWG-MRT criteria (2008), PV and ET diagnosis was reviewed according to the most recent WHO criteria. We performed time-to-event analysis with Cox regression models using either the time elapsed after ET or PV diagnosis or the time elapsed after SMF diagnosis, events being defined as the diagnosis of ST. Concomitant JAK inhibitor therapy was considered a dynamic (time-dependent) covariate present from the date of drug start. Likewise, the pre- and post-SMF periods were compared considering SMF as a time-dependent state. This study was approved by the Review Board of each Institution and conducted in accordance with the Declaration of Helsinki.
Results: Within 768 SMF, 394 were PET and 374 PPV MF. Median follow up time was 14.5 years (range, 0.9-45.9) from ET/PV diagnosis and 3.0 years (range, 0.6-27.3) from SMF diagnosis. We identified 71 patients (9.2% of the entire cohort) who developed a ST (included one multiple myeloma and four lymphoproliferative disorders). We excluded from the analysis myelodysplastic syndromes, acute leukemias, carcinomas in situ, breast fibroadenomas, superficial bladder carcinoma and non-melanoma skin cancers.
The most frequent (≥10%) ST subtypes were: breast (17 cases), prostatic (10) and kidney cancer (7). In 11 patients the date of ST occurrence was unreported and therefore they were excluded from time dependent analysis. As for the other 60 cases, 13 (21.7%) were diagnosed before ET/PV development, 22 (36.7%) during the ET/PV phase and 25 (41.6%) after SMF transformation.
The cumulative incidence of ST was 0.44% person-year of follow up for ET/PV developing SMF and 0.98% person-year of follow up for SMF. There was a trend of association between male gender and ST occurrence after ET/PV (P=0.054) and after SMF diagnosis as well (P=0.055). No other statistically significant differences in demographics, driver mutations, karyotype, bone marrow fibrosis, and MYSEC-PM strata were found at the time of SMF diagnosis between SMF patients with and without ST.
Then, we focused on 165 SMF patients treated with JAK inhibitors (of whom 10 during ET and 15 during PV phase): 128 received ruxolitinib, 11 fedratinib, 11 momelotinib, one XL019 and 14 JAK inhibitors sequentially. We did not find any correlation between JAK inhibitors treatment given at any time point of the follow-up and occurrence of ST (Log-rank P=1). Of note, the four patients with lymphoma did not receive JAK inhibition.
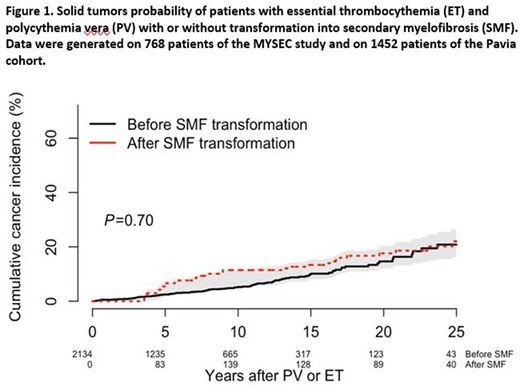
In the Pavia cohort, within a median follow up of 4.7 years (range, 0.6-39.7), 24 (3.9%) PV and 40 (4.8%) ET patients developed a ST. The incidence of ST in the Pavia dataset was 0.74% person-year of follow up. We eventually merged the MYSEC and the Pavia cohorts. As for the latter dataset we can not exclude SMF evolution with a longer follow-up, we treated SMF occurrence of the merged group as a time dependent covariate. The probability of developing ST was similar in the group of patients evolved into SMF and in those who did not (P=0.7, Figure 1).
Conclusions: This study provides evidence that: 1) the cumulative incidence of ST is about 1% person-year of follow up in SMF patients; 2) JAK inhibitors given during ET/PV or SMF phase are neutral for ST development within the limit of current follow up; 3) developing SMF in patients with PV or ET does not imply a higher risk of ST. These findings highlight the need of studies aimed at identifying patients at higher risk of ST occurrence.
Rambaldi:Roche: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Italfarmaco: Consultancy; Omeros: Consultancy; Amgen Inc.: Consultancy; Pfizer: Consultancy. Komrokji:Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding. Gotlib:Kartos: Consultancy; Promedior: Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Blueprint Medicines: Consultancy, Honoraria, Research Funding; Deciphera: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Kiladjian:Celgene: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cervantes:Hospital Clinic Barcelona: Employment; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees. Devos:Celgene: Consultancy; Novartis: Consultancy; Takeda: Consultancy. Palandri:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Passamonti:Janssen: Consultancy, Speakers Bureau; Roche: Consultancy; Novartis: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal