Abstract
Introduction. The combination of lenalidomide and rituximab induces response in patients (pts) with both treatment-naïve (TN) and relapsed (R) chronic lymphocytic leukemia (CLL). In order to identify the pts who benefit from this treatment, we conducted a phase II study to prospectively evaluate how clinical characteristics, prognostic factors and gene mutations correlated with response and survival in pts with TN and R CLL treated with this combination.
Methods. Treatment with lenalidomide started on day 9 of cycle 1 at 10 mg orally and administered daily. Rituximab, 375 mg/m2, was administered intravenously every 28 days for a total of 12 cycles. All pts had clinical staging, laboratory and pathological characteristics determined prior to treatment initiation. A SureSelect custom panel of 295 genes was performed in pretreatment bone marrow samples. The primary endpoint was overall response rate (ORR) and responses were assessed according to IW-CLL 2008 criteria at month 3, 6 and every 6 months thereafter. The secondary endpoints were treatment safety and association of baseline characteristics and gene mutations with response.
Results. One-hundred and twenty pts were enrolled in this study, 61 with TN and 59 with R CLL. Baseline characteristics are summarized in the Table. Twenty-one out of 54 (39%) TN pts and 18 out of 53 (34%) R pts had more than 1 mutation. Among TN pts, NOTCH1 mutations significantly associated with BCOR mutations (p=0.02) and SPEN mutations (p=0.02); among R pts NOTCH1 mutations significantly associated with XPO mutations (p=0.01) and MGA mutations (p=0.001), and TP53 mutations significantly associated with advanced Rai stage (p=0.01) and beta-2-microglobulin (B2M) > 4 mg/L (p=0.02). The frequency of single gene mutations did not significantly differ between the 2 groups.
ORR was 73% for TN pts and 64% for R pts; complete remission was achieved in 35% of TN pts and 28% of R pts, with MRD eradication in 16% and 2% of pts, respectively.
The association among all baseline characteristics, gene mutations and ORR was evaluated. Among TN pts, B2M < 4 mg/L was associated with higher ORR on univariate analysis (85% vs 55%, p=0.03). Among R pts, age < 65 years (78% vs 50%, p=0.05), Rai stage 0-II (79% vs 48%, p=0.03), B2M < 4 mg/L (84% vs 46%) and estimated glomerular filtration rate ≥ 60 mL/min (76% vs 38%, p=0.01) were associated with higher ORR on univariate analysis; on multivariate analysis (MVA), only B2M maintained its association with ORR (odds ratio 0.2; 95% confidence interval [CI] 0.1-0.9; p=0.03)
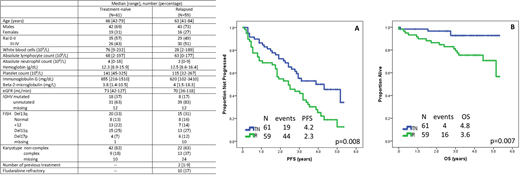
After a median follow-up of 41 months (range 1-62), 45 (74%) TN pts and 52 (88%) R pts interrupted treatment, and median PFS was 50 months (95% CI 31-69 months) and 28 months (95% CI, 17-39 months), respectively (Figure). On MVA, age ≥ 65 years (hazard ratio [HR] 5; 95% CI 1.4-21; p=0.02), KRAS mutation (HR 40; 95% CI 1.2-1000; p=0.04), SPEN mutation (HR 20; 95% CI 1.5-250; p=0.02) and ASXL1 mutation (HR 13; 95% CI 1.2-125; p=0.03) associated with shorter PFS among TN pts. SPEN mutation (HR 21; 95% CI 2.1-200; p=0.01) and FBXW7 mutation (HR 12; 95% CI 1.2-125; p=0.03) associated with shorter PFS among R pts.
At most recent follow-up, 4 TN pts (2 on study) and 16 R pts (5 on study) have died, and median OS has not been reached (Figure). Two TN and 5 R patients died while on study. Causes of death among TN pts included infection in 1 pt and 2nd primary neoplasm (SPN) in 1 pt. Among R pts, they included infection in 3 pts, SPN in 1, and sudden death of unknown etiology 1 pt. On MVA analysis TP53 mutation was associated with shorter OS in TN pts, and FISH positive for del17p/del11q was associated with a trend for shorter OS in R pts.
Grade 3-4 neutropenia was the most common toxicity, observed in 5% of cycles in TN pts and 17% in R pts, median duration of neutropenia was 7 days (range, 7-14 days) among TN pts, and 10 days (range, 7-14 days) among R pts.
Discussion. The combination of lenalidomide and rituximab is an effective and safe regimen for the treatment of pts with TN and/or R CLL. B2M is the only predictive factor of response to this regimen in both in TN and R pts. Gene mutations inducing increased NOTCH1 signaling, such as SPEN and FBXW7 mutations, predicted shorter PFS after treatment with lenalidomide and rituximab.
Thompson:Adaptive Biotechnologies: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees. Daver:Incyte: Research Funding; Pfizer: Research Funding; Alexion: Consultancy; BMS: Research Funding; Karyopharm: Research Funding; Karyopharm: Consultancy; Incyte: Consultancy; Otsuka: Consultancy; ImmunoGen: Consultancy; Daiichi-Sankyo: Research Funding; Novartis: Research Funding; Pfizer: Consultancy; Sunesis: Research Funding; Novartis: Consultancy; Sunesis: Consultancy; ARIAD: Research Funding; Kiromic: Research Funding. Jain:Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; Seattle Genetics: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Infinity: Research Funding; Genentech: Research Funding; Astra Zeneca: Research Funding; Abbvie: Research Funding; Verastem: Research Funding; ADC Therapeutics: Research Funding; Pharmacyclics: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; BMS: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; ADC Therapeutics: Research Funding; Celgene: Research Funding; Pfizer: Research Funding; Astra Zeneca: Research Funding; Abbvie: Research Funding; Pfizer: Research Funding; Servier: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Genentech: Research Funding; Cellectis: Research Funding; Cellectis: Research Funding; Incyte: Research Funding; Pharmacyclics: Research Funding; Infinity: Research Funding; Seattle Genetics: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. O'Brien:Celgene: Consultancy; Gilead: Consultancy, Research Funding; Acerta: Research Funding; Pfizer: Consultancy, Research Funding; Astellas: Consultancy; Janssen: Consultancy; GlaxoSmithKline: Consultancy; Sunesis: Consultancy, Research Funding; Regeneron: Research Funding; Pharmacyclics: Consultancy, Research Funding; Vaniam Group LLC: Consultancy; TG Therapeutics: Consultancy, Research Funding; Kite Pharma: Research Funding; Amgen: Consultancy; Alexion: Consultancy; Aptose Biosciences Inc.: Consultancy; Abbvie: Consultancy. Wierda:Genentech: Research Funding; AbbVie, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal