Abstract
Introduction: Extranodal natural killer/T-cell lymphoma (ENKTL) is an aggressive form of non-Hodgkin's lymphoma,which is relatively more common in Asia and Latin America.Radiation therapy(RT) is widely administered for patients with localized nasal disease. However, local and systemic failures are observed frequently in patients who receive RT alone.Therefore, chemotherapy is needed in combination with RT to reduce the risk of recurrence. Concurrent chemoradiotherapy (CCRT) is expected to improve both local and systemic disease control and has been established as a standard therapy for several types of solid tumors. Therefore,we conducted a phase II study of pegaspargase-based CCRT to explore a more effective treatment for localized nasal natural killer (NK)/T-cell lymphoma.
Methods: Enrolment began in May 2016 for a phase II single-center clinical trial. Chemotherapy and RT were simultaneously started within 7 days after registration. The drug administration schedule were as follows: day1,deep intramuscular injection of 2000 unit/m2 pegaspargase at three different sites. Chemotherapy was planned to repeat every 3 weeks. Pegaspargase were administered only if the plasma fibrinogen level was greater than 0.75g/L. Six courses of chemotherapy were planned.All patients received three-dimensional (3D) conformal radiation therapy by using 4 or 6MV photons generated from a linear accelerator. The involved-field radiation (IFRT) dose was 50 Gy, which was given as 2.0 grays (Gy) per daily fraction with 5 weeks.
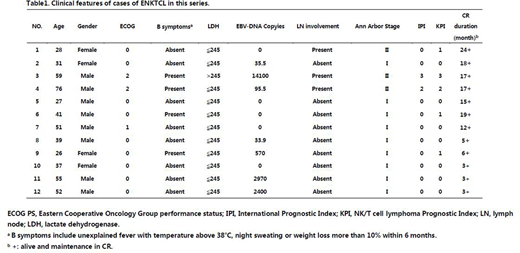
Results: Until May 2, 2018, we have completed the enrolment of 12 patients with newly-diagnosed untreated ENKTL. The main clinical characteristics of the 12 patients are presented in Table 1. All patients (n=12) have begun treatment and are evaluable for response. Of the 12 patients, complete remission (CR) rates is 100% to concurrent chemoradiotherapy alone. None of patients has experienced disease progression until now. Toxicities are recorded as the number of patients experiencing a certain adverse event.The most common grade 1-2 non-haematological (non-heme) adverse effects (AEs) are fatigue (n=2), nausea (n=1),oral mucositis (n=1) and increased transaminases (n=1).Eight (n=8) patients were confirmed to have decreased fibrinogen. Most of these were grade 1/2 and no bleeding or other adverse events were observed.There was no grade 3/4 non-heme toxicities during treatment. Grade 1-2 hematological (heme) AEs included neutropenia (n=6), anemia (n=5) and thrombocytopenia(n=2).Grade3-4 haematological AEs was anemia (n=1). No patients interrupted treatment for severe adverse events and no treatment-related deaths occurred.
Conclusions: Preliminary data indicate that pegaspargase with concurrent radiotherapy in newly diagnosed ENKTL patients was efficacious and well-tolerated, while the long-term outcome is required to be followed up.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal