Abstract
Introduction: Genetic alterations at 9p24.1 resulting in overexpression of programmed death-1 (PD-1) ligands are near-universal in cHL (Roemer et al, J Clin Oncol 2016); cHL may thus be uniquely sensitive to PD-1 blockade. Nivolumab (nivo), an anti-PD-1 monoclonal antibody, was associated with an objective response rate (ORR) of 69% in relapsed/refractory (R/R) cHL after auto-HCT and irrespective of prior brentuximab vedotin (BV) in the phase 2 CheckMate 205 study (Armand et al, J Clin Oncol 2018; NCT02181738). Whether some patient (pts) can derive very long clinical benefit, whether depth of response predicts long-term outcome, whether treatment (Tx) can be interrupted in complete remission (CR), and whether the safety profile of nivo in R/R cHL changes with prolonged Tx all remain unclear. We therefore present an updated analysis of CheckMate 205, focusing on long-term efficacy and safety.
Methods: This international, single-arm, multi-cohort study enrolled pts aged ≥18 y with R/R cHL after auto-HCT. Pts were BV naïve (Cohort A), had prior BV failure after auto-HCT (Cohort B), or had received BV before and/or after auto-HCT (Cohort C). Pts received nivo 3 mg/kg every 2 wk until disease progression (PD)/unacceptable toxicity. Pts in Cohort C discontinued nivo after 1 y in CR and could resume if they relapsed within 2 y of the last dose. Primary endpoint was ORR per independent radiology review committee (IRC); additional endpoints included duration of response (DOR) per IRC, progression-free survival (PFS) per IRC, overall survival (OS), and safety. Time to next Tx (TTNT: time from first dose to next systemic Tx or death) was an exploratory post-hoc analysis. Responses were assessed using International Working Group 2007 criteria.
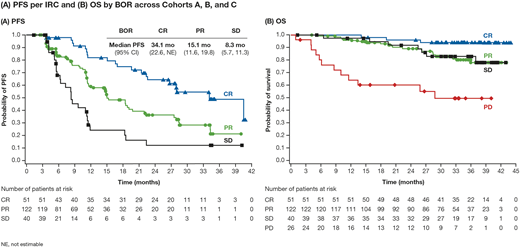
Results: In total, 243 pts were enrolled to Cohorts A (n=63), B (n=80), and C (n=100). Baseline characteristics have been previously described (Armand et al, J Clin Oncol 2018). At data cut-off, minimum follow-up was 31 mo and 49 pts (20%) were still on Tx; the most common reason for discontinuation was PD (35%). Median duration of Tx was 14 mo. ORR per IRC was 71% (65%, 71%, 75% in Cohorts A, B, C, respectively) with a best overall response (BOR) of CR in 21% (32%, 14%, 20% in Cohorts A, B, C, respectively). Among 51 pts who achieved CR, 20 had CR as the first response and 31 improved from partial remission (PR), mostly (n=28/31) within 1 year of first PR. Median time to response was 2 mo, and to CR was 4 mo. Within the first 6, 12, and 18 mo, 68%, 71%, and 71% of pts, respectively, achieved a response. Median DOR was 18 mo overall, and was 32 and 13 mo in pts with a BOR of CR and PR, respectively. Among responders, 64%, 44%, 31%, and 21% of pts had a DOR of at least 6, 12, 18, and 24 mo, respectively. Overall, 11 pts in Cohort C discontinued with persistent investigator-assessed CR; 2 reinitiated nivo due to PD. Median (95% CI) PFS per IRC among all pts was 15 (11-19) mo (Figure A), and was 17, 12 and 15 mo in Cohorts A, B, and C, respectively. Median OS was not reached in any cohort; 24-mo OS rates were 90%, 86%, and 86% in Cohorts A, B, and C, respectively, and were similar among pts in CR, PR, or with stable disease (SD; Figure B). Median TTNT was 29, 27, and 20 mo in Cohorts A, B, and C, respectively. The most common Tx-related adverse events (AEs) of any grade (G) were fatigue (24%), diarrhea (16%), and rash (12%), each <1% G3-4. Tx-related infections were reported in 15% of pts, 2% G3-4. Tx-related infusion related reactions were reported in 34 pts (14%), <1% G3-4. The most commonly reported immune-mediated AE category was rash, which was G3-4 in 4 pts (2%). In total, 26 pts (11%) experienced an AE of any cause leading to discontinuation. There were no Tx-related deaths.
Conclusions: With the longest phase 2-3 study follow-up of a checkpoint inhibitor in R/R cHL to date, nivo was associated with frequent and durable responses regardless of BV Tx history. More than 1 in 5 responders remained in response ≥2 years later. With extended follow-up, additional pts achieved CR in all cohorts. Pts with CR had longer PFS than pts with PR or SD. However, pts with both PR and SD had prolonged OS, unlike pts with PD, which may suggest that clinical benefit duration is not well predicted by conventional response criteria. Nivo continued to be well tolerated, with no new safety signals. Characteristics of long-term responders will be presented.
Study support: BMS. Medical writing: A Gill, Caudex, funded by BMS
Armand:Infinity: Consultancy; Affimed: Consultancy, Research Funding; Pfizer: Consultancy; Otsuka: Research Funding; Adaptive: Research Funding; Merck: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Roche: Research Funding; Tensha: Research Funding. Younes:Celgene: Honoraria; Genentech: Research Funding; Janssen: Honoraria, Research Funding; Bayer: Honoraria; Novartis: Research Funding; J&J: Research Funding; Astra Zeneca: Research Funding; BMS: Honoraria, Research Funding; Merck: Honoraria; Abbvie: Honoraria; Takeda: Honoraria; Seattle Genetics: Honoraria; Roche: Honoraria, Research Funding; Sanofi: Honoraria; Incyte: Honoraria; Curis: Research Funding; Pharmacyclics: Research Funding. Zinzani:PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Honoraria, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees. Collins:ADC Therapeutics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Speakers Bureau; Celleron: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Speakers Bureau; Celgene Corporation: Research Funding; BMS: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria; Amgen: Research Funding. Ramchandren:Merck: Research Funding; Bristol-Myers Squibb: Consultancy; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics LLC an AbbVie Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Cohen:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BioInvent: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Takeda: Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Infinity Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees. De Boer:Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Membership on an entity's Board of Directors or advisory committees; EISA: Membership on an entity's Board of Directors or advisory committees. Kuruvilla:Lundbeck: Honoraria; Gilead: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Princess Margaret Cancer Foundation: Research Funding; Leukemia and Lymphoma Society Canada: Research Funding; Abbvie: Consultancy; Merck: Consultancy, Honoraria; Karyopharm: Honoraria; Roche: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Amgen: Honoraria; Celgene: Honoraria. Trněný:F. Hoffman-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board, Research Funding; Sandoz: Honoraria; Gilead: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Abbvie: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Morphosys: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board. Shipp:Merck: Research Funding; Bayer: Research Funding; AstraZeneca: Honoraria; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sacchi:Bristol-Myers Squibb: Employment, Equity Ownership. Sumbul:Bristol-Myers Squibb: Employment. Ansell:Trillium: Research Funding; Pfizer: Research Funding; Regeneron: Research Funding; Celldex: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; Merck & Co: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal