Abstract
Background: Small molecule drugs that target specific pathogenic gene mutations offer the possibility of treating cancers while maintaining a wide therapeutic margin. Potential mechanisms of resistance to such targeted therapies include alternate or additional mutations in the target gene, activating mutations in genes downstream in the same pathway, or mutations in genes in an alternate prosurvival pathway. Co-occurring mutations that contribute to resistance may be present at the start of therapy and lead to immediate treatment failure. Therapy planning might be optimized if one could predict failure to a specific treatment even when the target was present. Ivosidenib (IVO, Agios Pharmaceuticals, Inc.) and enasidenib (ENA, Celgene Corp.) are small molecule inhibitors of IDH1 and IDH2 gain-of-function mutant enzymes, respectively, developed for treatment of acute myeloid leukemia (AML) with such mutations. In the Phase 1 monotherapy trials of IVO and ENA for treatment of relapsed or refractory (R/R) AML, the majority of patients appeared to be resistant to treatment; 67% and 77% of the patients with the target IDH mutation, respectively, failed to achieve complete remission with full (CR) or partial (CRh) hematological recovery. IDH1- and IDH2-mutated AMLs have multiple co-occurring mutations which may impact this resistance. The purpose of this study was to determine if there is a co-occurring mutation signature at treatment baseline that can identify patients with relapsed or refractory IDH1- or IDH2-mutated AML who would be resistant to treatment with mutant IDH inhibitors. We hypothesized that since the molecular pathogenesis was the same in IDH1- and IDH2-mutated AML (overexpression of the oncometabolite 2-hydroxyglutarate ), the co-occurring mutations that contributed to resistance would also be the same.
Methods: Adequate clinical and genomics data were available for 147 patients with IDH1-mutated R/R AML treated with IVO (Study AG120-C-001, NCT02074839) and 87 patients with IDH2-mutated R/R AML treated with ENA (Study AG221-C-001, NCT01915498). Only patients with the treatment-targeted IDH mutations as determined by the companion diagnostic were included in the analysis cohorts. Patients who failed to achieve an FDA-adjudicated CR or CRh within the first 6 cycles of therapy were considered nonresponders; patients without a response assessment after start of therapy were excluded from analysis. Genomics data were generated using next-generation sequencing platforms.
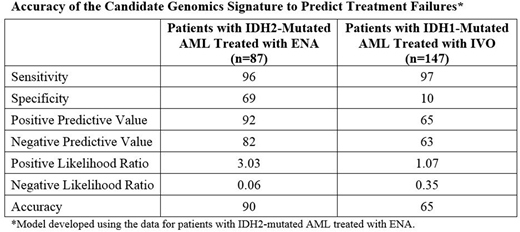
Results: To address the hypothesis, a mutational signature or model was first generated with the data from the ENA-treated patients, and subsequently tested on the data from the IVO-treated patients. In the first step, data were fit to the model using a proprietary algorithm that implemented a Monte-Carlo simulation expansion of Fisher's regularized linear discriminant analysis. The candidate model with the minimal number of genes that can differentiate more than half of the difference between responder and nonresponder groups includes 19 genes. However, more accuracy was attained by developing a 46- or 96-gene model. By Ingenuity Pathway Analysis, nonresponders differed from responders by mutations in only a few functional groups of genes (leukemia cell proliferation, leukemia cell differentiation and leukemia cell movement). In the assessment of diagnostic accuracy, the 96-gene model had 96% sensitivity, 69% specificity and 90% accuracy for identifying patients with IDH2-mutated AML who would not respond to treatment with ENA (Table). When tested in the cohort of patients with IDH1-mutated AML treated with IVO, the model had 97% sensitivity but only 10% specificity and 65% accuracy.
Conclusions: We conclude that genomics data may be useful to identify patients with co-occurring mutations that predict resistance to targeted therapies, but a predictive genomics signature may be specific to the treatment target gene rather than being generalizable across target genes that share a common mechanism of pathogenesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal