Abstract
INTRODUCTION: R-CHOP is effective for diffuse large B-cell Lymphoma (DLBCL), but many patients (Pts) relapse or have refractory disease, likely due to inherent biologic differences in DLBCL subtype. Activated B-Cell (ABC) subtype DLBCL signals through Nuclear Factor-κ-B (NF-κB) and is more likely to display treatment failure than DLBCL arising from the germinal center (GC). Proteasome inhibitors disrupt NF-κB signaling, but randomized trials have failed to demonstrate clinical benefit of adding bortezomib to R-CHOP for the treatment of non-GC DLBCL. Carfilzomib (Car) displays superior clinical activity relative to bortezomib in plasma cell neoplasms and, while occasionally associated with cardiac events, does not have dose-limiting neuropathy. To explore the safety and efficacy of Car in upfront treatment of DLBCL, we initiated a phase I/II clinical trial of Car + R-CHOP and report the phase I results.
METHODS: 24 adult (age ≥ 18) Pts with untreated de novo or transformed DLBCL, adequate organ function and performance status were enrolled. During 3 x 3 dose escalation, Car was given at 20 mg/m2 on days 1 and 2, with R-CHOP on day 2 for 6 cycles (n = 6). Due to grade 4 thrombocytopenia, the protocol was amended to administer Car at a dose (in mg/m2) of 20 on days 1 and 2 of cycle 1 with rituximab (R) on day 2 and CHOP on day 3, followed by a Car dose of 20 (n=3), 27 (n=3), 36 (n=3), 45 (n=3) and 54 (n = 6) on days 1 and 2 of cycles 2-6. All Pts received pegfilgrastim the day after CHOP and zoster prophylaxis with acyclovir x 6 months post treatment. Echocardiograms were obtained at baseline and at conclusion of therapy to assess the cardiac safety of combining Car with anthracycline. Interim response assessments with CT +/- PET were performed after cycle 3 and end-of-treatment response assessments were uniformly captured with PET.
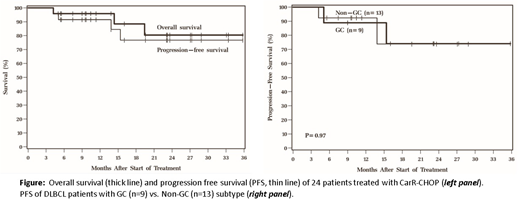
RESULTS: The median age was 57 (range 24-77) years old. 63% of patients were female. Stage at diagnosis was I-II (58%) or III-IV (32%). The majority of Pts had ECOG performance status of 0-1 (88%). B symptoms were present in 21% of Pts and 54% had an increased LDH at diagnosis. 29% had >1 extranodal site. IPI score was 0-1 (50%), 2 (21%) or 3-4 (39%). For this phase I dose escalation study, eligible Pts included primary mediastinal lymphoma (n = 1) and DLBCL of GC (n = 9), non-GC (n = 13) and unknown (n = 1) Hans algorithm subtypes. Hematologic adverse events (AEs) included 60 grade 1/2, 27 grade 3 and 16 grade 4 AEs. Grade 3/4 hematologic toxicities included neutropenia (n=14), thrombocytopenia (n = 6) anemia (n = 6), with only 4 cases of grade 3 febrile neutropenia. Grade 3/4 non-hematologic AEs were generally consistent with known R-CHOP toxicity were notable for: hypertension (n = 2), decreased ejection fraction (n =2), GI hemorrhage (n = 2) dizziness, headache, and syncope (n = 1 each), thromboembolic event (n=1), hyperglycemia (n=2), increased ALT (n=1) and nausea/vomiting (n=2). Compared to age-matched controls, end-of-treatment echocardiograms of CarR-CHOP treated Pts showed no statistically significant additional effect on ejection fraction (EF) [94.8% vs. 90.0% of pre-treatment value, respectively (P = 0.19)] after 6 cycles of treatment and there was no association of change in EF with Car dose (P = 0.61). There were no dose limiting toxicities. As of June 2018, median follow-up among surviving Pts was 16 months. There were 3 deaths during the study period, 2 from lymphoma and 1 from lung cancer. The overall response rate was 92% [75% complete remission (CR), 17% partial remission]. 18-month Kaplan Meier estimates of PFS and overall survival were 77% and 88%, respectively (Figure). There was no significant difference in CR rates or PFS for patients with GC vs. non-GC subtype (P = 0.65 and 0.61, respectively).
CONCLUSION: CarR-CHOP is safe at a recommended phase II dose of 20 mg/m2 on day 1 & 2 for cycle 1 followed by 56 mg/m2 for cycles 2-6, without significant excess cardiac effects. Within the limitations of a prospective phase I clinical trial with potential patient selection bias, preliminary efficacy data suggest a high complete metabolic response rate and equivalent outcomes for patients with GC and non-GC subtype. Phase II accrual is ongoing for non-GC DLBCL only and additional correlative studies of the molecular subtype of DLBCL will be incorporated into future analysis.
Hill:Amgen: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Tomlinson:Foundation Medicine: Consultancy. Caimi:Genentech: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Celgene: Speakers Bureau; Kite Pharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal