Abstract
Background:
High throughput sequencing of immunoglobuline heavy chain gene rearrangements (IGH) recently demonstrated that B-cell precursor ALL (BCP-ALL) is a highly oligoclonal disease in children (Theunissen, Hematologica, 2017), however, little is known about the degree of oligoclonality in adults with BCP-ALL. Furthermore, no data exist on potential changes of the subclonal composition during therapy. Therefore, we aimed to monitor the clonal architecture of the leukemic blasts before and after Rituximab containing induction treatment in the context of the current German Multicentric Acute Lymphoblastic Leukemia (GMALL) 08/2013 trial.
Materials & Methods:
We identified and studied complete and incomplete immunoglobulin (IG) heavy chain rearrangements in 100ng of bone marrow (BM) DNA at diagnosis (dx) and 500ng BM DNA after Rituximab-containing induction I in 19 patients with BCP-ALL. We employed IGH-VJ FR1 and IGH-DJ NGS assays developed within the EuroClonality-NGS Consortium (www.euroclonalityngs.org), performing next generation sequencing on Illumina MiSeq. We analysed data with the ARResT/Interrogate bioinformatics platform (Bystry, Bioinformatics, 2017), which is also able to isolate and use the DN-J stem of V/DJ junctions to link clonally related rearrangements. Clones with abundance ≥5 %, and all subclones carrying the same DN-J stem as the dominant clones were considered as leukemia-associated and studied further. Employing the sequence of the DN-J stem, we could also link related complete and incomplete rearrangements, even though those are amplified in two separate PCRs.
Results:
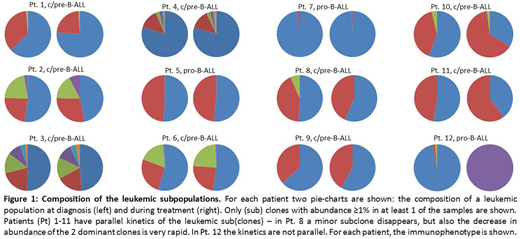
At dx, 18/19 patients carried at least 1 IGH rearrangement with abundance ≥ 5%, 10 patients carried 2 or more. In all 18 patients with dominant markers detected at dx, subclones with abundance <5% carrying the same DN-J stem as the dominant clone were present, indicating oligoclonality and clonal evolution. On average, 53.1 (range 0-295) (sub)clones per DN-J stem were detected at dx, and 32.7 (range 0-238) after treatment.
Next, we compared the kinetics of all (sub)clones with abundance ≥1% in at least one of the time-points. Of the 18 patients, 6 only had subclones with abundance <1%, and we did not investigate the kinetics of such subclones. In another 11 patients (Fig. 1, Pt. 1-11), all sub(clones) had the same kinetics, with no clone gaining predominance over time. In 1 patient (Fig. 1, Pt. 12), 3 (sub)clones which were present at dx disappeared and a new subclone appeared after treatment. This patient had a pro-B immunophenotype, where oligoclonality and clonal instability are well known phenomena (Szczepanski, Leukemia, 2001).
Conclusions:
It has recently been shown that ALL is a highly oligoclonal disease in children (Theunissen, Hematologica, 2017), and our study extends this finding to adults with BCP-ALL. We furthermore demonstrate that the subclonal composition remains stable in the majority of patient during induction chemoimmunotherapy. The fact that the response to treatment is generally consistent among different (sub)clones has important implications for MRD quantification as it reassures the usage of the dominant clonal IG gene rearrangement for MRD monitoring in ALL. However, also significant changes of the clonal composition may occur in BCP-ALL as exemplified in one patient of our cohort. Further investigations are necessary to elucidate factors that influence subclonal heterogeneity in response to treatment.
Viardot:Pfizer: Consultancy, Honoraria; Gilead Kite: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Amgen: Consultancy; BMS: Consultancy, Honoraria. Kneba:AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Goekbuget:Pfizer: Consultancy, Other: Travel support, Research Funding; Novartis: Consultancy, Other: Travel support, Research Funding; Kite / Gilead: Consultancy; Amgen: Consultancy, Other: Travel support, Research Funding; Celgene: Consultancy. Brüggemann:Incyte: Consultancy; PRMA: Consultancy; Regeneron: Research Funding; Affimed: Research Funding; Pfizer: Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau; Roche: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal