Key Points
Early consolidation with BV improves 5-year PFS vs best supportive care and reduces the need for subsequent therapy.
At 5-year follow-up, BV shows long-term tolerability, with peripheral neuropathy continuing to improve and/or resolve.
Abstract
The phase 3 AETHERA trial established brentuximab vedotin (BV) as a consolidative treatment option for adult patients with classical Hodgkin lymphoma (cHL) at high risk of relapse or progression after autologous hematopoietic stem-cell transplantation (auto-HSCT). Results showed that BV significantly improved progression-free survival (PFS) vs placebo plus best supportive care alone. At 5-year follow-up, BV continued to provide patients with sustained PFS benefit; 5-year PFS was 59% (95% confidence interval [CI], 51-66) with BV vs 41% (95% CI, 33-49) with placebo (hazard ratio [HR], 0.521; 95% CI, 0.379-0.717). Similarly, patients with ≥2 risk factors in the BV arm experienced significantly higher PFS at 5 years than patients in the placebo arm (HR, 0.424; 95% CI, 0.302-0.596). Upfront consolidation with BV significantly delayed time to second subsequent therapy, an indicator of ongoing disease control, vs placebo. Peripheral neuropathy, the most common adverse event in patients receiving BV, continued to improve and/or resolve in 90% of patients. In summary, consolidation with BV in adult patients with cHL at high risk of relapse or progression after auto-HSCT confers a sustained PFS benefit and is safe and well tolerated. Physicians should consider each patient’s HL risk factor profile when making treatment decisions. This trial was registered at www.clinicaltrials.gov as #NCT01100502.
Introduction
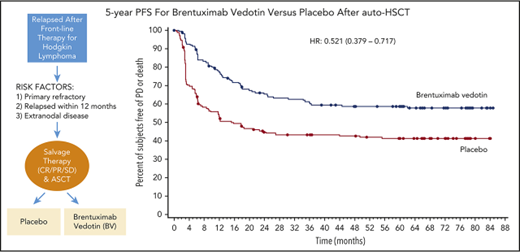
The standard of care for patients with relapsed or refractory Hodgkin lymphoma (HL) is high-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation (auto-HSCT). Approximately 50% of patients can be cured after auto-HSCT; however, most patients with unfavorable risk factors progress after transplantation and historically have had poor outcomes, including a median survival of 2.4 years from auto-HSCT and 1.2 years from auto-HSCT if relapse occurs <1 year from auto-HSCT.1,2
The phase 3 AETHERA trial of brentuximab vedotin (BV) consolidation after auto-HSCT in patients with classical HL at high risk of relapse or progression established BV as a therapeutic option in this setting.3 After a median observation of 30 months, BV plus best supportive care resulted in significant improvement in progression-free survival (PFS) vs placebo plus best supportive care (placebo; hazard ratio [HR], 0.57; P = .001). BV was safe and generally well tolerated; the most common treatment-emergent adverse events in the BV arm were peripheral neuropathy (PN; 67%), neutropenia (35%), and upper respiratory tract infection (26%). Here we present an updated analysis at 5 years of follow-up.
Methods
AETHERA is a global phase 3 randomized placebo-controlled trial; the full trial design and methodology have been previously published.3 Briefly, eligible BV-naive patients with HL must have undergone auto-HSCT before randomization and have been at high risk of relapse after auto-HSCT based on having either relapsed or progressive HL that occurred <12 months from the end of frontline therapy; a history of refractory HL, defined as progression during or failure to achieve a complete remission after frontline therapy; or extranodal involvement at the time of pre–auto-HSCT relapse. As previously reported,3 patients provided written informed consent in accordance with the Declaration of Helsinki, and the study was approved by the institutional review board at each study site.
A total of 329 patients were randomized to receive 1.8 mg/kg of BV or placebo once every 3 weeks for up to 16 cycles starting 30 to 45 days after auto-HSCT. Statistical procedures were followed as previously published3 ; the primary end point was PFS as assessed by independent review. A post hoc analysis of PFS in patients with ≥2 (n = 280) or ≥3 (n = 166) of any of the following risk factors was also conducted: relapse <12 months or refractoriness to frontline therapy; best response of partial response or stable disease to most recent salvage therapy; extranodal disease at pre–auto-HSCT relapse; B symptoms at pretransplantation relapse; or ≥2 prior salvage therapies. Stratified HRs according to the number of risk factors are provided.
A post hoc time to second subsequent therapy (TTSST) analysis was also performed; events were defined as death resulting from any cause or receipt of 2 subsequent HL-directed therapies after completing assigned study treatment. Patients without a TTSST event were censored at the time of their last follow-up visit.
Here we present data at 5-year follow-up, with a data cutoff of 15 November 2017. All follow-up data were investigator reported.
Results and discussion
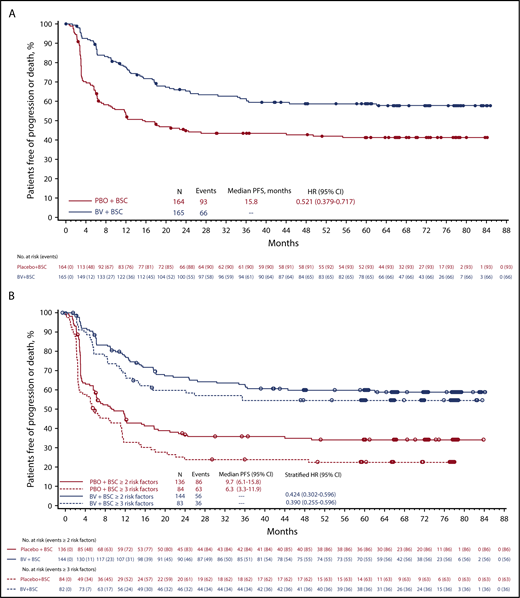
As previously reported, 165 and 164 patients were randomized to the BV and placebo arms, respectively.3 At 5-year follow-up, patients randomized to BV continued to experience significantly longer PFS than patients who received placebo. The median 5-year PFS with BV was not reached; it was 15.8 months with placebo. The 5-year PFS rate was 59% (95% confidence interval [CI], 51-66) with BV vs 41% (95% CI, 33-49) with placebo (HR, 0.521; 95% CI, 0.379-0.717; Figure 1A).
Five-year PFS. PFS at 5 years per investigator (A) and in patients with ≥2 or ≥3 risk factors (B). BSC, best supportive care; PBO, placebo.
Five-year PFS. PFS at 5 years per investigator (A) and in patients with ≥2 or ≥3 risk factors (B). BSC, best supportive care; PBO, placebo.
At 3-, 4-, and 5-year follow-up, patients who received BV consistently maintained the ∼30% reduction in PFS events over patients who received placebo (30%, 28%, and 30% fewer events, respectively). The benefit of BV was more pronounced in patients with additional pre–auto-HSCT risk factors; the 5-year PFS HR was 0.424 (95% CI, 0.302-0.596) in patients with ≥2 risk factors and 0.390 (95% CI, 0.255-0.596) in those with ≥3 (Figure 1B). Although there was no PFS difference between arms in patients with 1 risk factor, this group comprised <15% of the cohort, and the small number per arm precluded any conclusion for these patients.
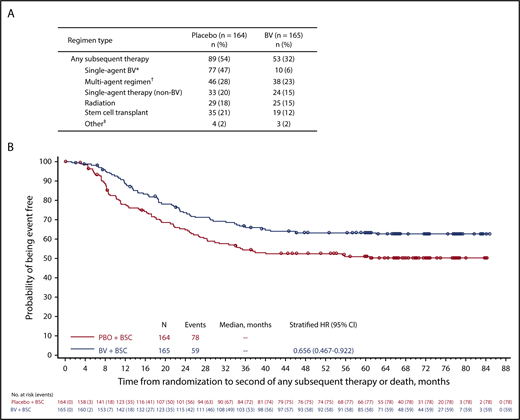
Consistent with the observed differences in progression, significantly fewer patients treated with BV received subsequent anticancer therapy (32%; n = 53) compared with patients treated with placebo (54%; n = 89; P < .0001). Of these, fewer patients treated with BV vs those treated with placebo received 1 (n = 11 vs 21), 2 (n = 17 vs 21), or ≥3 (n = 25 vs 47) lines of subsequent therapy. Notably, 87% of patients in the placebo arm received single-agent BV as a subsequent therapy (Figure 2A).
Subsequent therapy. Summary of any new subsequent antitumor therapy (A) and probability of events and time to death or second subsequent therapy (B). *Among 10 patients in the BV arm who were subsequently re-treated with BV, the overall response rate was 60% (including 4 complete responses, 2 partial responses, 1 stable disease, 1 progressive disease, and 2 unknown). †Includes multiagent regimens that include BV (n = 2 in each treatment group). ‡Includes donor lymphocyte infusion (n = 2 in each group) and unknown (n = 2 in placebo group; n = 1 in BV group). BSC, best supportive care.
Subsequent therapy. Summary of any new subsequent antitumor therapy (A) and probability of events and time to death or second subsequent therapy (B). *Among 10 patients in the BV arm who were subsequently re-treated with BV, the overall response rate was 60% (including 4 complete responses, 2 partial responses, 1 stable disease, 1 progressive disease, and 2 unknown). †Includes multiagent regimens that include BV (n = 2 in each treatment group). ‡Includes donor lymphocyte infusion (n = 2 in each group) and unknown (n = 2 in placebo group; n = 1 in BV group). BSC, best supportive care.
At 5 years, 40 and 37 deaths were reported in the BV and placebo arms, respectively. Because post–auto-HSCT therapies have afforded patients prolonged survival, an analysis of overall survival is not planned until 2020. Instead, an analysis of TTSST was performed, based on scientific guidance from the European Medicines Agency,4 to provide a measure of ongoing disease control in a patient population at high risk of relapse where multiple additional therapies are likely to be used. At 5 years, 36% and 46% of patients in the BV and placebo arms, respectively, had received ≥2 subsequent therapies for HL or had died (HR, 0.656; 95% CI, 0.467-0.922; Figure 2B). Consistent with these findings, the need for subsequent HSCT was lower in the BV arm (12%; n = 19) than in the placebo arm (21%; n = 35). These data demonstrate a reduction in the need for subsequent therapy in patients who receive BV as consolidation after first auto-HSCT, even when most patients in the placebo arm received subsequent BV at the time of relapse.
At the latest follow-up, 90% of patients with PN in the BV arm reported resolution or improvement, including 73% with complete resolution. The median time to resolution or improvement of PN in the BV arm was 37.6 weeks vs 9.1 weeks in the placebo arm. Similarly, of the 95 patients (n = 76 in BV arm, n = 19 in placebo arm) with treatment-emergent PN who remained in remission for >5 years without further treatment, 95% in each arm had resolution or improvement of PN. Of 14 patients still on study in the BV arm with ongoing PN, 10 have a maximum of grade 1, 3 have a maximum of grade 2, and 1 has a maximum of grade 3; assessment of resolution in the patient with grade 3 PN is confounded by the presence of radiation myelitis.
Secondary malignancies occurred in 6 and 3 patients in the BV and placebo arms, respectively; they included myelodysplastic syndromes (n = 2), acute myelogenous leukemia (n = 1), and pancreatic, lung, and bladder cancers (n = 1 each) in the BV arm and mantle cell lymphoma, acute myelogenous leukemia, and myelodysplastic syndromes in the placebo arm (n = 1 each).
In summary, consolidation with BV in patients with classical HL at high risk of relapse or progression after auto-HSCT showed sustained PFS benefit vs placebo ∼5 years after the last patient was randomized. The clinical benefit was more pronounced in patients with ≥2 or ≥3 risk factors. Patients who received BV as early consolidation maintained a PFS benefit at 5 years and, despite a high rate of subsequent BV therapy in the placebo arm after relapse, also had a longer TTSST. Patients who received BV consolidation also required fewer therapies, including subsequent transplantations. Lastly, PN continued to improve and/or resolve in 90% of patients. A final analysis of overall survival is expected in 2020.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Medical editorial assistance was provided by Ann T. Yeung (ScientificPathways, Inc.), with funding from Seattle Genetics, Inc.
Authorship
Contribution: C.H.M., J.S., and A.S. contributed to the concept and design of the study; C.H.M. and T. McClendon contributed to the analysis of the data; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: C.H.M. reports receiving research support from Seattle Genetics and SAB; M.H.A. reports receiving honoraria for speaker engagements from Seattle Genetics; V.B. reports receiving funding from Gamida Cell and scientific advisory fees from Kite Pharma, Inc.; S.V. reports receiving research funding from Takeda and advisory honoraria from Takeda International, Takeda Italia, and Italfarmaco; J.W. reports receiving advisory fees from Takeda, Janssen-Cilag, Roche, Celgene, Amgen, Bristol-Myers Squibb, and Incyte, research/educational grants to his institution from Roche, Janssen-Cilag, Takeda, and GlaxoSmithKline/Novartis, honoraria for speaker engagements from Roche, Takeda, Celgene, Servier, and Janssen-Cilag, and travel grants from Roche and Gilead; T. Masszi reports receiving consultancy fees from AbbVie, Bristol-Myers Squibb, Janssen-Cilag, Novartis, Pfizer, and Takeda; A.S. reports receiving honoraria for speaker engagements from Takeda and scientific advisory honoraria from MSD Pharmaceuticals and Bristol-Myers Squibb; T. McClendon was a paid consultant for Seattle Genetics, Inc., at the time of this writing; J.L. is an employee of Seattle Genetics, Inc.; and C.L. was an employee of Takeda Pharmaceuticals Ltd during development of this manuscript. The remaining authors declare no competing financial interests.
Correspondence: Craig H. Moskowitz, Cancer Service Line, Sylvester Comprehensive Cancer Center, University of Miami Health System, 1475 NW 12th Ave, Miami, FL 33136; e-mail: chm78@miami.edu.