In this issue of Blood, Liao et al report that anemic stress induces CCL2 production in the spleen, which recruits monocytes that associate with stress erythroid progenitors (SEPs) and differentiate into central macrophages to create erythroblastic islands (EBIs).1
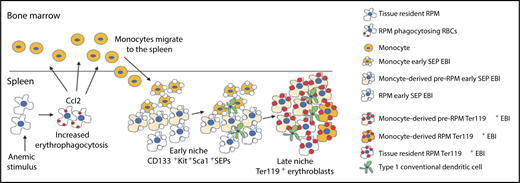
EBIs in stress erythropoiesis. Anemic stimulus induces erythrophagocytosis and production of the chemokine CCL2 by splenic RPMs, which attract monocytes into spleen. These monocytes associate with CD133+KIT+ early SEPs and undergo stepwise maturation into pre-RPMs and RPMs. Within EBIs, monocyte maturation occurs in concert with maturation of SEPs such that the majority of Ter119+ mature erythroblasts are associated with pre-RPMs and RPMs. Also seen within EBIs are conventional type 1 dendritic cells. RBCs, red blood cells. See Figure 7J in the article by Liao et al that begins on page 2580.
EBIs in stress erythropoiesis. Anemic stimulus induces erythrophagocytosis and production of the chemokine CCL2 by splenic RPMs, which attract monocytes into spleen. These monocytes associate with CD133+KIT+ early SEPs and undergo stepwise maturation into pre-RPMs and RPMs. Within EBIs, monocyte maturation occurs in concert with maturation of SEPs such that the majority of Ter119+ mature erythroblasts are associated with pre-RPMs and RPMs. Also seen within EBIs are conventional type 1 dendritic cells. RBCs, red blood cells. See Figure 7J in the article by Liao et al that begins on page 2580.
Almost all tissues in the body harbor resident macrophages that help maintain local tissue homeostasis.2 Examples include surfactant recycling by alveolar macrophages and iron recycling by splenic red pulp macrophages (RPMs). At the steady state, most tissue-resident macrophages originate from embryonic precursors and are largely maintained by local proliferation.3 However, tissue injury, infection, or inflammation leads to an influx of monocytes that undergo local differentiation into macrophages.3 Whether tissue-infiltrating monocyte-derived macrophages can take up “permanent residency” and become functionally redundant with preexisting local embryonic macrophages is a matter of ongoing debate and likely depends on the context.
Erythropoiesis occurs in specialized structures known as EBIs, which feature a centrally located macrophage (central macrophage) surrounded by developing erythroid cells at various stages of differentiation.4 Central macrophages support erythropoiesis by providing nutrients and other factors to surrounding erythroblasts and phagocytose nuclei extruded from developing erythrocytes.4 Like most tissue-resident macrophages, central macrophages at the steady state are thought to originate from embryonic precursors. However, their ontogeny during erythropoiesis induced by anemic stress is unclear. Murine stress erythropoiesis occurs in the spleen and liver (extramedullary) and utilizes SEPs that are distinct from erythroid progenitors at the steady state.5 Preexisting splenic macrophages may proliferate to generate new central macrophages, as suggested by Ulyanova et al.6 Alternatively, circulating monocytes may be recruited to differentiate into central macrophages. This distinction is important from the standpoint of understanding both tissue macrophage ontogeny and stress erythropoiesis.
In this issue of Blood, Liao et al examine EBI formation in the spleen and explore the origin of central macrophages using Cre-LoxP–based lineage tracing and bone marrow transplantation in multiple murine models of stress erythropoiesis induced by acute anemia. The authors find that anemic stress increases erythrophagocytosis and promotes CCL2 production by splenic RPMs. Increased CCL2 leads to the recruitment of circulating monocytes, which become associated with SEPs to form new EBIs in the spleen. Notably, the authors find that newly recruited monocytes associate with immature SEPs and subsequently differentiate into mature central macrophages in concert with the maturation of SEPs. In other words, monocytes and SEPs appear to “co-differentiate” in stress-induced EBIs (see figure). These findings provide important insights into how the EBI microenvironment develops during stress erythropoiesis.
RPMs regulate erythroid turnover in the spleen by removing old and damaged erythrocytes as well as by interacting with developing erythroblasts. A previous study showed that monocytes can differentiate into pre–red pulp macrophages (pre-RPMs) and RPMs, which are driven by heme-mediated induction of the transcription factor SpiC.7 Liao et al find that monocytes associated with EBIs in stress erythropoiesis also undergo sequential maturation into pre-RPMs and RPMs. Consistent with this, the authors observe increased SpiC expression in stress-induced EBIs. A previous study showed that depletion of CD169-expressing cells, which includes both RPMs and pre-RPMs, disrupts stress erythropoiesis, highlighting the importance of these cells in EBIs.8 In contrast, SpiC-deficient mice do not display overt defects in stress erythropoiesis.6 A likely explanation, as the authors point out, is that SpiC deficiency selectively depletes RPMs but not pre-RPMs. Consistent with this, the authors observe that the majority of stress-induced EBIs in the spleen are associated with pre-RPMs and not RPMs. Nonetheless, future analyses of stress-induced EBIs in SpiC-deficient mice may reveal additional functions of mature RPMs in this context.
An intriguing observation by Liao et al is the detection of a subset of dendritic cells, cDC1 (type 1 conventional dendritic cells), within EBIs. Dendritic cells are ontologically and functionally distinct from macrophages.9 They specialize in antigen presentation, but a previous report showed that cDC1 cells can stimulate stress erythropoiesis by producing stem cell factor (ligand for KIT receptor).10 Although the mechanistic details of how cDC1 senses anemic stress and regulates stress erythropoiesis are unclear, their presence in EBIs is consistent with a role of dendritic cells in this process and suggests that myeloid components of EBIs are more heterogeneous than previously appreciated. Future work will likely provide more insight into this aspect of stress erythropoiesis.
In summary, Liao et al demonstrate that monocytes and SEPs associate to form EBIs de novo during stress erythropoiesis, underscoring the dynamic nature of the stress erythropoiesis niche. Subsequently, monocytes mature into central macrophages in concert with surrounding SEPs within the EBI microenvironment. That being said, it remains unclear whether differentiation of the surrounding SEPs regulates differentiation of the central monocyte/macrophage or vice versa. It will also be important to examine the relevance of these findings in anemia of chronic inflammation. Future work addressing these issues and building on Liao et al’s report will further extend our understanding of stress erythropoiesis and EBI development.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal