Abstract
Andexanet alfa, a reversing agent for anticoagulants that inhibit factor Xa, has recently been licensed in the United States. We discuss the impact of this licensure on current practice and review in detail the problems of a neglected and growing clinical area: reversing the anticoagulation effect of factor Xa inhibitors in bleeding trauma patients. We identify areas of practice that need research so that care of bleeding trauma patients receiving direct factor Xa inhibitors can be improved.
The challenge
The introduction of direct oral anticoagulants (DOACs) without specific reversal agents caused concern within the trauma community, which, after the release of dabigatran, initiated an urgent call for reversal agents to manage traumatic hemorrhage in patients receiving DOACs.1 The global licensing of idarucizumab for the reversal of dabigatran and the recent approval by the US Food and Drug Administration (but not as yet by the European licensing agency) of andexanet alfa (Andexxa) are therefore welcomed.2
Not only can andexanet alfa reverse the anticoagulant effects of DOACs that directly inhibit factor Xa (rivaroxaban, apixaban, edoxaban, and betrixaban), but it is also the first reversing agent for low molecular weight heparin (LMWH) and fondaparinux, which both inhibit factor Xa indirectly through antithrombin. DOACs account for a growing proportion of oral anticoagulants prescribed. Their main indication at present, despite growing use in the long-term prevention of both venous and arterial thromboembolisms, is the prevention of stroke in those with atrial fibrillation. Atrial fibrillation is increasingly seen as a result of our ageing population, with incidence rising with each decade, from 0.5% in those age 50 to 59 years to almost 9% in those age 80 to 90 years.3 So how have we managed to this point without a reversing agent for direct oral factor Xa inhibitors? Firstly, there is an overall lower risk of intracranial hemorrhage compared with vitamin K antagonists (VKAs).4 Secondly, because of the shorter half-life of DOACs compared with VKAs, their effect wears off quickly, so reversal is often not required. Lastly, there has been widespread use of prothrombin complex concentrates (PCCs) to reverse DOACs that inhibit factor Xa, a practice that has evolved despite limited evidence5-7 and increased thrombin generation seen after use in healthy volunteers.8
The question regarding the clinical efficacy of andexanet remains: will reversal of direct inhibitors of factor Xa in those bleeding actually improve clinical outcomes? In the catastrophic setting of intracranial hemorrhage (ICH) in those prescribed VKAs, although randomized controlled trial data show faster time to correction of international normalized ratio with 4-factor PCCs when compared with fresh frozen plasma,9 it remains unclear whether clinical outcomes improve.10-14 This probably reflects the extensive cerebral damage present before the bleed is recognized, although authors of 1 study felt swifter PCC use after ICH would lead to less enlargement of the hematoma.10 Interestingly, preliminary data from trauma patients have shown conflicting clinical outcomes and mortality between those who received DOACs compared with those receiving warfarin. One retrospective study showed improved clinical outcomes with DOACs, 1 prospective study showed no difference, and another prospective study in patients with traumatic brain injury showed poorer outcomes with DOACs compared with warfarin.15-17
Andexanet alfa
Andexanet alfa is described as a decoy molecule, a catalytically inactive form of factor Xa, which binds to inhibitors of factor Xa (currently including rivaroxaban, apixaban, edoxaban, betrixaban, LMWH, and fondaparinux) and reverses their anticoagulant activity. The supporting clinical data are based mainly on reversing the effects of rivaroxaban and apixaban in the clinical situations of gastrointestinal bleeding and ICH in a nonrandomized cohort study.18-20 More than 90% of the anticoagulant effects of factor Xa–inhibiting agents were quickly reversed, and clinical control of hemostasis was deemed to be good or excellent in 79% of patients receiving andexanet alfa.20 Higher dosing of andexanet alfa is required for recent ingestion.20,21 As yet, there are no data on the utility of andexanet alfa in those with renal and/or hepatic failure. Andexanet alfa binds and sequesters factor Xa in the intravascular space, restoring thrombin generation, and anti-Xa activity returns to the normal decay curve after the infusion has ended. Importantly, the DOAC may still be present in the plasma after the effect of andexanet alfa has worn off.17,18 When healthy volunteers were administered andexanet alfa to reverse the effects of rivaroxaban and apixaban, there were transient increases in prothrombin fragment 1+2 and D-dimer afterward. This may be related to the binding of andexanet alfa to tissue factor pathway inhibitor, because trials of concizumab, an inhibitor of the tissue factor pathway, showed similar changes.19 Of greater concern, Connolly et al20 found that after reversal of DOACs in bleeding patients, there was an 18% rate of thrombosis and 15% rate of death in the first 30 days, although the absence of a control group makes these results difficult to interpret. It is unclear whether these thrombotic events reflect the patient’s underlying thrombotic risk and/or whether the additional use of andexanet alfa also has a prothrombotic effect.
Trauma and major bleeding
Trauma in elderly patients is rising and is a worldwide problem,22 with hemorrhage remaining the cause of 40% of potentially preventable trauma deaths.23 Acute traumatic coagulopathy is present immediately after injury in 25% to 35% of trauma patients,24 and the coagulopathy will be further aggravated by prior use of an anticoagulant. Acute traumatic coagulopathy is defined in part by prolongation of prothrombin time (PT) and viscoelastic hemostatic assays24 and is associated with poor outcomes.24,25 Management of coagulopathy is key in trauma hemorrhage, and most trauma guidelines recommend early use of tranexamic acid, fresh frozen plasma, and platelets.26-31 The pathophysiology of acute traumatic coagulopathy involves activation of coagulation, fibrinolysis, and the protein C pathway, along with endotheliopathy and platelet dysfunction.32,33 Importantly, thrombin generation is preserved in most patients with acute traumatic coagulopathy.32-34 The use of viscoelastic hemostatic monitoring to guide the use of blood components has been shown to reduce bleeding and transfusions of red cells, plasma, and platelets and to potentially reduce mortality.35
Our options in reversal of direct inhibitors of factor Xa
With the arrival of andexanet alfa for reversal of major bleeding in patients receiving DOACs with factor Xa inhibitor activity, acute and emergency care providers together with hemostasis experts will need to learn for whom and when andexanet alfa is indicated. Although it may seem intuitive that the drug, marketed as an antidote, will be used for reversal of all major bleeding events in patients receiving DOACs, targeting the right patients and implementing andexanet alfa in clinical practice may not be so simple.
By necessity, in the absence of an antidote for the reversal of direct oral inhibitors of factor Xa, anticoagulation reversal has evolved against the background of a changing approach to management of acute traumatic coagulopathy. It is important to note that in central Europe, despite poor supporting evidence, guidelines suggest considering the use of PCCs in the immediate resuscitation of patients with traumatic coagulopathy in preference to the use of fresh frozen plasma in all patients, not just those receiving anticoagulation drugs before hospital admission.31,36
The use of PCCs has also been shown to reduce prolonged PT and increase thrombin generation in volunteers receiving rivaroxaban,37,38 apixaban,39,40 and edoxaban.41 Clinically, PCCs have emerged as the recommended standard for treating acute bleeding in patients receiving DOACs that inhibit factor Xa, as stated by an Expert Consensus Decision Pathway by the American College of Cardiology7 and European Heart Rhythm Association,42 despite limited evidence.43 PCCs reduce major bleeding and the laboratory anticoagulation effect of inhibitors of factor Xa in patients with ICH and gastrointestinal bleeding, with clinical efficacy ranging from 65% to 69%.44,45 The later rate of thromboembolic events has been studied by Schulman et al,44 who found an 8% incidence of major thromboembolic events after PCC use for major bleeding related to DOACs that inhibit factor Xa. Majeed et al45 identified 2 ischemic cerebrovascular events within 1 week of PCC administration in 84 patients with major bleeding. Thus, thromboembolic events after PCC use to reverse DOACs that inhibit factor X seem to be infrequent, although comparison groups are lacking.
PCCs have been used in retrospective series to treat traumatic coagulopathy in those receiving DOACs,46,47 although neither the clinical efficacy nor the rate of thromboembolic events has been well described. Therefore, although andexanet alfa now provides the long-desired antidote for DOACs with factor Xa inhibition, the use of PCCs as prohemostatic agents for managing traumatic bleeding raises interesting questions regarding the choice between andexanet alfa and PCCs in patients with traumatic bleeding receiving factor Xa inhibitors. Of note, the ANNEXA-4 trial only included patients with nontraumatic bleeding.20 A major gap in the present understanding is the lack of published research on use of andexanet alfa to reverse direct inhibitors of factor Xa before urgent surgery and in severely injured patients. Furthermore, there are no trials comparing PCCs and andexanet alfa in this setting.
Measuring and monitoring anti-Xa effect
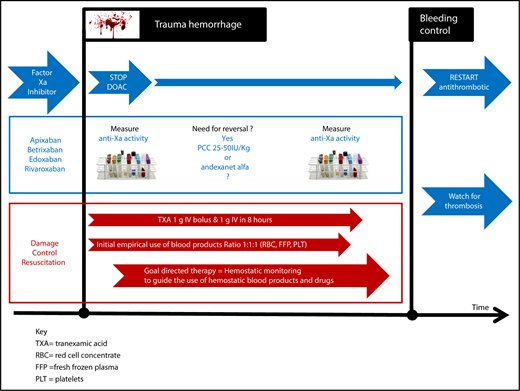
Direct oral inhibitors of factor Xa can each prolong both PT and activated partial thromboplastin time (APTT) variably, with the added complication of apixaban producing unpredictable prolongation of PT.15 Therefore, monitoring of the anticoagulation activity of a DOAC in a bleeding trauma patient is complex, where prolongation of PT and APTT can occur as a result of acute traumatic coagulopathy. PT and APTT are of little value in the discrimination between acute traumatic coagulopathy and DOAC anticoagulation, supporting the need for more specific discriminative assays. The anti-Xa assays of DOACs with factor Xa inhibition18 has not been validated in those with acute traumatic coagulopathy, raising concerns over the interpretation of their efficacy.48 Perhaps the worst situation for a trauma physician is an unconscious trauma patient who is known to be taking an oral anticoagulant, but there is uncertainty as to which anticoagulant. How should this patient be managed (Figure 1)? Conventional testing with PT, APTT, and Clauss fibrinogen will not be able to differentiate between the effects of DOACs or traumatic coagulopathy or the relative contribution of both. Currently, in such a patient, urgent anti-Xa activity is required, and the ability of anti-Xa assays to detect both the effects of LMWH and direct inhibitors of factor Xa will be an advantage.49 However, the urgency of clinical decision making for DOAC reversal after injury makes waiting for these results impractical. Fast turnaround times for anti-Xa assays and a close working partnership between trauma physicians and their hemostasis experts will be vital in the management of such patients. There has been a suggestion that viscoelastic hemostatic assays might help, and research is ongoing.50 One potential future advancement is a new urinary dipstick assay, which has approval in Europe and is awaiting US Food and Drug Administration review. It identifies DOACs in urine, although it cannot provide quantitative data.51
Proposed hemostatic management of a patient receiving an anti-Xa DOAC who has traumatic bleeding.
Proposed hemostatic management of a patient receiving an anti-Xa DOAC who has traumatic bleeding.
How to manage major hemorrhage in trauma patients receiving direct factor Xa inhibitors
Major bleeding in this situation should, in general, follow the standard hemorrhage protocol. Special attention to measuring the presence and plasma level of DOACs in the hemostasis laboratory as quickly as possible is advisable; however, until there is point-of-care testing, decision making on DOAC reversal will need to occur based on clinical assessment of the bleeding and anticoagulation history, not on laboratory testing. Reversal of direct oral factor Xa inhibitors should be used if bleeding is life threatening and can be done with titrated use of 25 to 50 IU/kg of a PCC, although the optimal reversal strategy for PCCs is uncertain.6,7 The potential increased risk of later thromboembolic complications should be remembered.8 Alternatively, andexanet alfa can be considered, although there are no approvals or data supporting its use in this setting. The price of reversal may also be considered, because the price of andexanet alfa has been set in the United States at ∼$58 000 for the high dose,52 although lower doses were used to treat 90% of patients studied in nontraumatic bleeding. In comparison, the cost of idarucizumab for dabigatran reversal is $4200, although its use in trauma patients also remains unstudied and may also carry a thrombotic risk.
Perspectives
Taken together, these observations suggest the urgent need for more research on the monitoring and reversal of the anticoagulant effect of direct inhibitors of factor Xa in bleeding trauma patients. Well-designed head-to-head randomized trials comparing andexanet alfa with PCCs in traumatic bleeding would be helpful to inform future practice. In the interim, it is important that hemostasis laboratories work with trauma departments to expedite turnaround times for DOAC plasma levels. Novel DOAC detection assays are urgently required in the management of bleeding trauma patients.
Authorship
Contribution: All authors jointly wrote the paper, contributing equally.
Conflict-of-interest disclosure: M.D.N. has had work funded by Janssen. The remaining authors declare no competing financial interests.
Correspondence: Beverley J. Hunt, Thrombosis & Haemophilia, St Thomas’ Hospital, Westminster Bridge Rd, London SE1 7EH, United Kingdom; e-mail: beverley.hunt@gstt.nhs.uk.