In this issue of Blood, Morales-Ortíz et al establish triggering receptor expressed in myeloid cells (TREM)-like transcript-1 (TLT-1) as an independent risk factor for acute respiratory distress syndrome (ARDS), and they show in a murine model of acute lung injury (ALI) that TLT-1 mediates fibrinogen deposition in the lungs and facilitates platelet-neutrophil release during transmigration.1
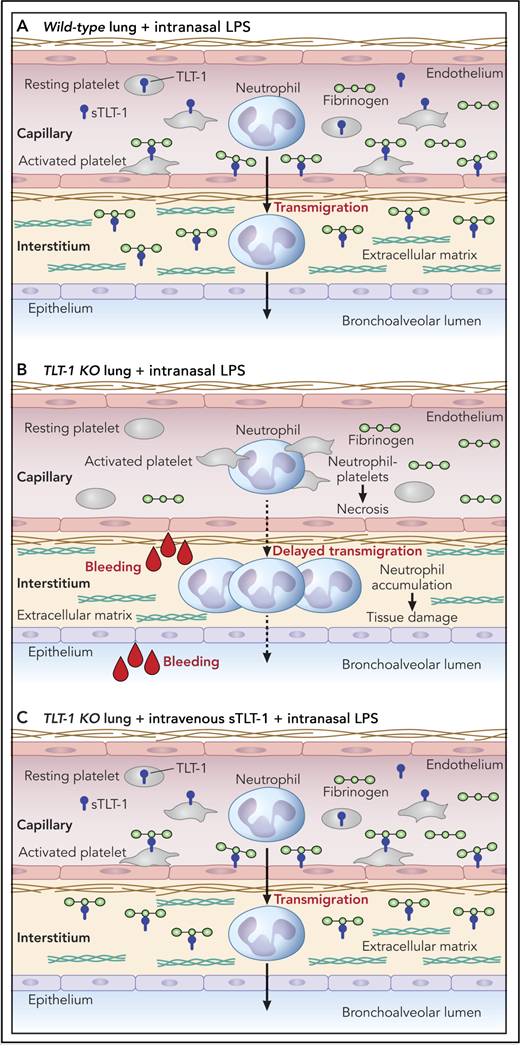
Schematic representation of lung response to intranasal lipopolysaccharide (LPS) treatment in (A) wild-type and (B) TLT-1 knockout (KO) mice. (C) Intravenous treatment with sTLT-1 rescues the LPS response in TLT-1 KO mice. Professional illustration by Patrick Lane, ScEYEnce Studios.
Schematic representation of lung response to intranasal lipopolysaccharide (LPS) treatment in (A) wild-type and (B) TLT-1 knockout (KO) mice. (C) Intravenous treatment with sTLT-1 rescues the LPS response in TLT-1 KO mice. Professional illustration by Patrick Lane, ScEYEnce Studios.
TLT-1 is a type-1 transmembrane protein that is highly and exclusively expressed in megakaryocytes and platelets.2-4 Structurally, it consists of a single immunoglobulin-variable domain in its extracellular region, a transmembrane domain, and a cytoplasmic tail containing 2 highly conserved tyrosine residues, 1 of which is found within an immunoreceptor-tyrosine-based inhibitory motif (ITIM) consensus sequence (I/V/LxYxxL/V, where x represents any amino acid), and the other is found within an ITIM-like sequence.5 Phosphorylation of these tyrosine residues provides a docking site for the Src homology-2 (SH-2) domain-containing tyrosine phosphatases Shp1 and Shp2 in activated platelets.3,4
TLT-1 is stored in α-granules and possibly in another platelet compartment, and it is rapidly upregulated to the surface of activated platelets.4,6 A soluble form of TLT-1 (sTLT-1) also exists, plasma levels of which are significantly elevated in sepsis.7 The majority of sTLT-1 is shed from the surface of activated platelets, and the remainder is an alternatively splicing isoform stored and released from α-granules. Washington et al7 previously showed that TLT-1 binds fibrinogen, which they further investigate and which features prominently in the Morales-Ortíz et al study. However, despite the high levels of TLT-1 on the surface of activated platelets, which binds fibrinogen, mice that lack TLT-1 exhibit only a minor bleeding diathesis, which raises this question: What is the physiological function of this highly abundant platelet fibrinogen receptor? The answer from Morales-Ortíz et al is that it regulates neutrophil transmigration in inflammatory conditions.
The hypothesis underpinning the study by Morales-Ortíz et al is that platelet TLT-1 regulates the progression of ALI/ARDS. The authors show retrospectively that ARDS patients with plasma levels of sTLT-1 >1200 pg/mL have a 91% increased risk of mortality compared with patients with <1200 pg/mL. Patients with <80 000 platelets/μL or coagulation failure had significantly lower survival rates than those with >80 000 platelets/μL and intact coagulation. These parameters did not, however, maintain significance when controlled for other covariates, including age, ventilator volume, creatinine, and Acute Physiology and Chronic Health Evaluation III scores, whereas sTLT-1 levels did, providing a better independent predictor of mortality than either platelet count or coagulation failure. These findings suggest that platelet-derived sTLT-1 plays a role in the underlying mechanism of disease progression, which the authors explored through the use of a murine model of lipopolysaccharide (LPS)-induced ALI (see figure). TLT-1–deficient mice treated intranasally with LPS showed increase alveolar bleeding, delayed neutrophil transmigration, and accumulation in the lung interstitial tissue, all of which are associated with reduced fibrinogen deposition and increased pulmonary tissue damage compared with control mice expressing TLT-1. Intriguingly, the absence of TLT-1 on platelets resulted in significantly more platelet-neutrophil conjugates and correlated with increased neutrophil necrosis. The interpretation is that TLT-1 somehow releases platelets from neutrophils when they transmigrate into the interstitium through a mechanism that seems to involve platelets sticking to fibrinogen in a TLT-1–dependent manner, details of which remain ambiguous. Infusion of sTLT-1 intravenously into TLT-1–deficient mice was taken up and detected on the surface of platelets, and it rescued the ALI response in these mice.
Collectively, findings from the Morales-Ortíz et al study not only establish TLT-1 as a new prognostic indicator of ARDS but also identify an important new player in the progression of the condition. As in all good science, additional questions are raised, the most important being how TLT-1 regulates platelet-neutrophil interactions and transmigration, whether a counter-receptor of TLT-1 exists on neutrophils and the downstream signaling pathways triggered in both platelets and neutrophils following engagement, and what sTLT-1-fibrinogen conjugates bind to on endothelial cells and in the vessel wall. This study lays the foundation for further clinical-scientific investigation into the functional roles of TLT-1 in health and disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal