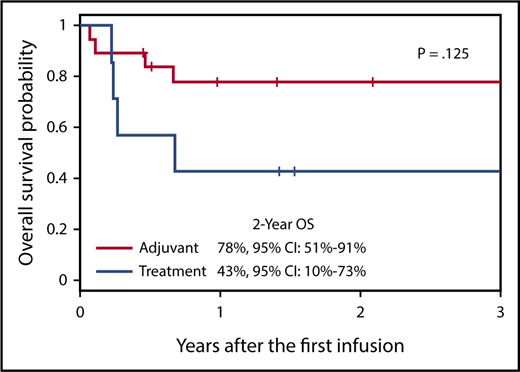
Patients with EBV-positive B- and T/NK-cell lymphoma or lymphoproliferative disorder were administered donor-derived T cells specific for the EBV LMP proteins following allogeneic HSCT, either as adjuvant therapy or as treatment of relapsed disease. The OS of patients receiving T cells as adjuvant therapy was 78%, supporting the infusion of LMP-Ts to improve the outcome of patients in this setting. CI, confidence interval. The figure has been adapted from Figure 7B in the article by McLaughlin et al that begins on page 2351.
Patients with EBV-positive B- and T/NK-cell lymphoma or lymphoproliferative disorder were administered donor-derived T cells specific for the EBV LMP proteins following allogeneic HSCT, either as adjuvant therapy or as treatment of relapsed disease. The OS of patients receiving T cells as adjuvant therapy was 78%, supporting the infusion of LMP-Ts to improve the outcome of patients in this setting. CI, confidence interval. The figure has been adapted from Figure 7B in the article by McLaughlin et al that begins on page 2351.
Adoptive transfer of EBV-specific cytotoxic T cells (EBV-CTLs) has proved highly successful in the prevention and treatment of EBV-driven posttransplant lymphoproliferative disease (PTLD) following HSCT.2 In these immunocompromised patients, the outgrowing B cells typically express all 9 of the EBV latent proteins, which constitute the target antigens of the T-cell response in healthy virus carriers.3 These same latent proteins are expressed in the in vitro EBV-transformed B-lymphoblastoid cell lines (LCLs) conventionally used to expand virus-specific T cells from the blood.3 Consequently, LCL-stimulated EBV-CTL preparations have broad EBV antigen specificity4 and can restore virus-specific T-cell immunity, leading to elimination of the proliferating EBV-positive B cells.2
In the present article, McLaughlin et al focus on the more challenging EBV-positive lymphomas arising in immunocompetent individuals. These tumors are far more difficult to target with adoptive T-cell therapy because viral protein expression is restricted to the less immunogenic latent proteins, including EBNA1, LMP1, and LMP2.3 T cells specific for these proteins are generally present at low frequencies in the blood and constitute only minor fractions of LCL-stimulated EBV-CTLs.4 To address this issue, McLaughlin et al use an elegant approach involving LMP1 and LMP2 overexpression in dendritic cells and LCLs to generate T-cell preparations enriched in LMP-specific T cells (LMP-Ts). Importantly, the authors have previously shown that autologous LMP-Ts can be administered safely and can induce durable responses in patients with relapsed/refractory EBV lymphoma.5
McLaughlin et al now assesses LMP-T infusion in the setting of allogeneic HSCT. Donor-derived LMP-Ts were administered to 26 patients who had undergone allogeneic HSCT for EBV-positive B-cell or T/NK-cell lymphoma or lymphoproliferative disorder. The prognosis of such patients is typically poor, particularly for those with T/NK-cell disease.6 LMP-Ts were infused either as adjuvant therapy in patients at high risk of relapse (n = 19) or as treatment of active disease (n = 7). Importantly, the allogeneic LMP-Ts were well tolerated, and only 1 dose-limiting toxicity occurred that was potentially attributable to T-cell infusion. The single incidence of de novo graft-versus-host disease was related to subtherapeutic immune suppression and quickly resolved.
Collectively, the 2-year overall survival (OS) was 68%. In line with data from the Center for International Blood & Marrow Transplant Research, OS was better for patients with B-cell (OS, 80%; n = 10) than T/NK-cell (OS, 60%; n = 16) disease. Nevertheless, increases in 2-year OS were seen in both disease subsets compared with historical cohorts, in which 2-year OS is 60% to 75% for B-cell-derived lymphomas and 30% to 50% for T/NK-cell-mediated diseases.6,7 Furthermore, OS was even higher in the subset of patients who received LMP-Ts as adjuvant therapy after allogeneic HSCT (OS, 78%; n = 19; see figure). These patients, who are at high risk of relapse, included 2 individuals with aggressive EBV-positive NK/T-cell lymphoma who remain disease free up to 3 years post–LMP-T infusion. Overall, this study clearly demonstrates the safety and efficacy of LMP-T infusion to lower the rate of relapse of EBV latency II B- and NK/T-cell lymphomas postallogeneic HSCT when administered as adjuvant therapy, offering hope to patients with these aggressive diseases.
The challenge now is to further enhance the success of LMP-T therapy in patients with relapsed or active disease at the time of infusion, where response rates were much lower (2-year OS, 43%; see figure).1 Optimizing T-cell therapy in this setting requires a deeper understanding of which individuals within these diverse patient groups respond to LMP-Ts and why others do not. Investigating the viral and immunological variables that influence patient outcome could uncover mechanistic insights for future treatment optimization. In this regard, McLaughlin et al provide some important clues from their in vitro analysis of the infused LMP-Ts. Because of the individualized nature of such treatments, the LMP-T preparations are unavoidably variable in their composition. Thus, although all products had in vitro reactivity against EBV, they differed considerably in their cellular constituents and in the diversity of LMP1 and LMP2 epitopes recognized. However, 2 interesting observations warrant further investigation: (1) responding patients generally received T-cell products containing higher LMP2 reactivity and maintained greater frequencies of LMP2-specific T cells in the blood, indicating the magnitude of the LMP2-specific response may be important; and (2) 2 patients who relapsed post–LMP-T administration were salvaged with nonselected donor lymphocyte infusion. Although it was not possible to evaluate EBV latent protein expression in the tumors of these 2 patients, this observation supports the development of T-cell therapies with a broader antigen-specific repertoire.1 Interestingly, previous analysis of polyclonal third-party EBV-CTLs used to treat PTLD has shown that the dominant antigen specificities present within the infused T cells does not necessarily correlate with clinical response.4 Thus, characterizing the T cells that expand in vivo, in combination with a comprehensive evaluation of EBV antigen expression in the tumor, may shed light on the precise specificities required for effective therapeutic responses.
Investigation of the tumor microenvironment may provide additional avenues to further improve LMP-T therapy for the EBV-positive B- and T/NK-cell lymphomas. EBV LMP1 has been shown to promote expression of the immune checkpoint inhibitor, programmed cell death ligand 1 (PD-L1),8 which engages the PD-1 receptor on T cells to inhibit signaling. Notably, PD-L1 expression has recently been reported in tumor biopsies from patients with EBV-positive B-cell8 and NK/T-cell9 lymphomas. Thus, in patients with active disease, the infused T cells may need to overcome local immunosuppression at the tumor site. Further characterization of the tumor microenvironment in future studies will indicate whether LMP-T therapy would benefit from coadministration of clinically available and emerging monoclonal antibodies targeting immune checkpoint inhibitors.10 Enhancing the in vivo efficacy of the infused LMP-Ts may further improve the outcomes for patients with relapsed EBV-positive B- and T/NK-cell lymphomas following allogeneic HCST.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal