In this issue of Blood, Arezes and colleagues present new and exciting evidence that erythropoietin (EPO) suppresses bone morphogenetic protein (BMP)-mediated signaling in an erythroferrone (ERFE)-dependent mechanism and ERFE in turn interferes with the binding of a specific set of BMPs to their receptors.1 These findings represent a breakthrough in the understanding of how EPO and ERFE function to decrease hepcidin expression.
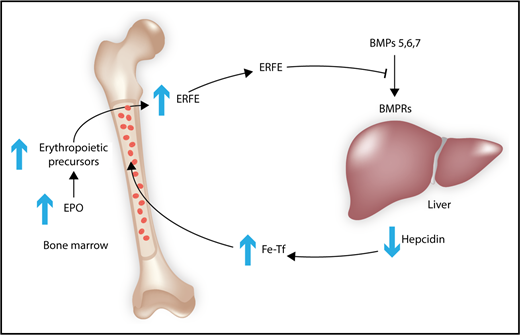
Model of the inhibition of hepcidin production by BMP6. Increased EPO expression leads to an expansion of erythroid precursors, which secrete ERFE, a hormone that circulates in the blood and that blocks the binding of BMPs 5, 6, and 7 to their BMP receptors. The decrease in BMP signaling in the liver leads to lower hepcidin production. Hepcidin negatively regulates the efflux of iron into the blood by downregulation of ferroportin. The lower hepcidin levels increase iron-bound Tf in the blood to promote the iron uptake of erythropoietic precursors to satisfy their needs for heme synthesis. BMPRs, BMP receptors; Fe-Tf, iron-loaded transferrin.
Model of the inhibition of hepcidin production by BMP6. Increased EPO expression leads to an expansion of erythroid precursors, which secrete ERFE, a hormone that circulates in the blood and that blocks the binding of BMPs 5, 6, and 7 to their BMP receptors. The decrease in BMP signaling in the liver leads to lower hepcidin production. Hepcidin negatively regulates the efflux of iron into the blood by downregulation of ferroportin. The lower hepcidin levels increase iron-bound Tf in the blood to promote the iron uptake of erythropoietic precursors to satisfy their needs for heme synthesis. BMPRs, BMP receptors; Fe-Tf, iron-loaded transferrin.
The present report provides the first mechanistic basis for the observation that EPO induction of erythropoiesis is associated with decreased hepcidin expression. The stimulation of erythropoiesis by EPO increases ERFE levels, a hormone secreted by erythroid precursors, and the demand for iron uptake and utilization.2 Hepcidin, a hormone secreted by hepatocytes, decreases iron in serum, thereby limiting iron availability for erythropoiesis. It acts by binding to and downregulating ferroportin. Ferroportin controls the iron levels in the serum. It is the iron exporter responsible for iron export out of macrophages and out of intestinal epithelial cells into the blood. Macrophages recycle iron from senescent red blood cells, and intestinal epithelial cells absorb iron from the diet. Hepcidin expression is largely controlled through the stimulation of the BMP-signaling pathway. The authors demonstrate that EPO treatment of ERFE–knockout mice severely blunts the suppression of hepcidin through a BMP-signaling pathway, thereby establishing a link between EPO and hepcidin.
Ever since the discovery that ERFE could decrease hepcidin expression in hepatocytes,2 the mechanism by which ERFE suppresses hepcidin has been sought. ERFE is a part of the tumor necrosis factor-β (TGF-β)-related family of proteins, and many anticipated that it would bind to a membrane-bound receptor on the surface of hepatocytes to antagonize hepcidin expression. In the present paper, the authors indirectly demonstrate that ERFE acts through the BMP-signaling pathway by interfering with the stimulation of BMP signaling by a specific set of BMPs (BMP5, BMP6, and BMP7), but has no effect on hepcidin induction by BMP 9 or activin B. BMP2, 4, 5, 6, 7, 9, and activin B all induce hepcidin expression via the BMP-signaling pathway in hepatoma cell lines (see figure).3,4 Previous studies also showed that the BMPs are predominantly expressed in the nonparenchymal cells of the liver, increasing the likelihood of serum ERFE being able to interfere with the endothelial-, Kupffer cell–, and stellate cell–derived BMPs.5,6
Although direct binding of ERFE to the subset of BMPs was not demonstrated, the authors use a blocking monoclonal antibody to ERFE that prevents ERFE’s ability to decrease hepcidin expression in cells stimulated by BMP5, BMP6, and BMP7. Interestingly, the monoclonal antibody competes with the BMP5, BMP6, and BMP7 for binding to ERFE. These BMPs are highly related and form 1 of the 7 groups of the BMP/growth-differentiation factor subfamily of the TGF-β superfamily.7 Their results strongly suggest that ERFE’s effect on hepcidin expression is by preventing these specific BMPs from binding to the BMP receptors on hepatocytes and raises the possibility that ERFE acts as an antagonist through a similar mechanism as Noggin, which binds to BMP7 and blocks BMP7-mediated signaling.
The story is not complete yet. BMP2, which falls into a different subgroup, is also required for full hepcidin expression,7,8 but the authors find that the monoclonal antibody does not inhibit the ability of ERFE to bind the antibody. These results imply that ERFE does not fully suppress hepcidin expression.
This work solves some of the questions in the regulation of iron homeostasis and raises new ones. Other mechanisms that control BMP2’s effect on hepcidin expression remain to be elucidated. Further studies are needed to determine whether the amount of ERFE in the blood is sufficient to block the BMPs from binding to BMP receptors. To date, a sensitive assay has been developed for ERFE9 and for BMPs,10 but the latter is based on transcriptional activation of reporter genes and therefore is not suitable to directly measure the serum levels of the different BMPs.
The current paper significantly enhances our understanding of the mechanism by which EPO indirectly suppresses hepcidin expression through the induction of ERFE. ERFE, in turn, increases iron availability for heme-synthesis in erythroid precursors by interfering with a subset of BMPs binding to their receptors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal