In this issue of Blood, Kho et al provide further evidence that platelets play a major role in the pathogenesis of malaria infection.1 In particular, they demonstrate that platelets can kill circulating parasites of all major Plasmodium species in human malaria. Elucidating the molecular mechanisms underpinning this platelet-directed killing mechanism may offer the opportunity to develop novel adjunctive antimalarial therapies.
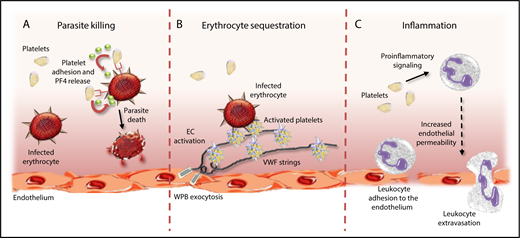
Platelets have multifactorial roles in malaria pathogenesis. Platelets can directly impact malaria pathogenesis through a number of different mechanisms. First, platelets can protect against malaria progression by binding to IEs and inducing Plasmodium killing through release of PF4 (A). In contrast, platelets tethered to ultralarge VWF strings secreted from activated ECs can promote cytoadhesion and sequestration of IEs, thereby promoting vascular occlusion (B). Finally, platelets can further promote malaria progression by driving proinflammatory effects directly, and by activating circulating leukocytes (C). WPB, Weibel-Palade body.
Platelets have multifactorial roles in malaria pathogenesis. Platelets can directly impact malaria pathogenesis through a number of different mechanisms. First, platelets can protect against malaria progression by binding to IEs and inducing Plasmodium killing through release of PF4 (A). In contrast, platelets tethered to ultralarge VWF strings secreted from activated ECs can promote cytoadhesion and sequestration of IEs, thereby promoting vascular occlusion (B). Finally, platelets can further promote malaria progression by driving proinflammatory effects directly, and by activating circulating leukocytes (C). WPB, Weibel-Palade body.
Human malaria continues to be a leading cause of mortality, with an estimated 500 000 deaths per year. Unfortunately, the majority of these deaths occur in sub-Saharan children under 5 years of age. In addition, it is important to recognize that malaria is also associated with significant global morbidity. For example, many children who survive cerebral malaria (CM) suffer secondary long-term neurologic sequelae. Despite this clinical burden, perhaps surprisingly, the biological mechanisms involved in the pathophysiology of severe malaria remain relatively poorly defined. Interestingly, thrombocytopenia is a common finding in humans and mice with malaria infection and is most marked in patients with severe Plasmodium falciparum infection.2 Moreover, previous studies have reported that extent of thrombocytopenia correlates with parasite density, severity of malaria infection, and clinical outcomes. Together, these data support the hypothesis that platelets are important in malaria pathogenesis. Critically, however, accumulating evidence suggests that the role of platelets in malaria is complex, and in particular, that platelets can impact upon malaria pathogenesis through a number of different mechanisms.
Previous postmortem studies on children with CM have demonstrated significant platelet accumulation in the cerebral microvasculature.3 This finding is consistent with other data demonstrating that platelets play a critical role in enabling cytoadhesion of malaria-infected erythrocytes (IEs) to endothelial cells (ECs). Interestingly, recent studies have defined a role for ultralarge von Willebrand factor (VWF) multimers in promoting this platelet-mediated IE cytoadhesion and proposed that this mechanism may be particularly important during the early stages of malaria infection.4 Platelets have also been reported to be important in enhancing IE clumping, which further contributes to microvasculature occlusion in malaria.5 In addition to these effects in promoting vasculature occlusion, platelets also play an important role in driving development of inflammation and EC activation, both of which are critical in malaria pathogenesis.2 Cumulatively, these data therefore suggest that the role of platelets in malaria pathogenesis is predominantly deleterious in nature, and that platelets promote malaria progression through a number of different routes (see figure).
In contrast, however, a number of studies have reported that platelets may actually have beneficial effects in protecting against malaria infection.2 In particular, a specific role for platelets in killing malaria parasites has been proposed. McMorran and colleagues previously showed that platelets could bind to human and murine IEs and significantly inhibit P falciparum growth in vitro.6 In addition, a direct role for platelet-mediated killing of intraerythrocytic malaria parasites was observed. Subsequent studies demonstrated that intraerythrocytic malaria killing by platelets required platelet-IE contact.6,7 Furthermore, platelet CD36, erythrocyte Duffy-antigen expression, and platelet factor-4 (PF4) were all identified as being important components in regulating the platelet killing phenomenon.7
Given the multifactorial mechanisms through which platelets can impact malaria pathogenesis, it is perhaps not surprising that previous studies have produced conflicting results about the importance of platelet-mediated effects.2,8 This variation is likely to be attributable in large part to differences in the experimental models and methodologies employed. Moreover, previous studies have also highlighted that there are significant differences in pathogenesis between human CM and experimental CM in murine models.9 In this context, the novel data presented by Kho et al are of particular relevance in that they report evidence of in vivo platelet-induced malaria killing in a large number of human patients naturally infected with malaria collected across 2 different geographical sites. Interestingly, although this platelet-killing effect was most marked in Plasmodium vivax patients, the effect was also observed across a number of other human Plasmodium species, including P falciparum, Plasmodium malariae, and Plasmodium knowlesi. Based upon their findings, the authors estimated that overall, platelets may kill up to 20% of circulating blood-stage Plasmodium in clinical malaria. In cases of P vivax infection, this platelet-killing phenomenon may actually account for as many as 60% of the total intraerythrocytic parasites.
These exciting novel human data on platelet-induced killing in human malaria raise a number of important clinical questions. Further studies will be required to define the biological mechanisms through which platelets recognize erythrocytes infected with different types of human malarial parasites. In addition, given the difference in clinical severity between different types of human malaria, it will be interesting to investigate the relative importance of platelet-mediated parasite killing for these different Plasmodium species. In view of the multiple roles played by platelets in modulating malaria pathogenesis, different aspects of platelet function may be more or less important in specific types of Plasmodium infection. Furthermore, it seems likely that the role played by platelets in malaria pathogenesis may vary at different times in the disease progression following infection. For example, it seems plausible that platelet-mediated malaria killing may be of particular importance in the early stages following malaria infection. Notwithstanding these questions, there is clearly an unmet clinical need to develop novel adjunctive therapeutic strategies to address the staggering global morbidity and mortality associated with human malaria. Consequently, elucidating the precise molecular mechanisms involved in regulating platelet-mediated killing of human malaria offers an exciting potential to uncover entirely new therapeutic avenues.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal