Key Points
Recruitment of neutrophils generates phospholipid oxidation and formation of CEP adducts with extracellular matrix proteins.
CEP–protein adducts form inflammatory-specific substrate for αMβ2 and αDβ2 integrin-mediated macrophage migration during inflammation.
Abstract
Early stages of inflammation are characterized by extensive oxidative insult by recruited and activated neutrophils. Secretion of peroxidases, including the main enzyme, myeloperoxidase, leads to the generation of reactive oxygen species. We show that this oxidative insult leads to polyunsaturated fatty acid (eg, docosahexaenoate), oxidation, and accumulation of its product 2-(ω-carboxyethyl)pyrrole (CEP), which, in turn, is capable of protein modifications. In vivo CEP is generated predominantly at the inflammatory sites in macrophage-rich areas. During thioglycollate-induced inflammation, neutralization of CEP adducts dramatically reduced macrophage accumulation in the inflamed peritoneal cavity while exhibiting no effect on the early recruitment of neutrophils, suggesting a role in the second wave of inflammation. CEP modifications were abundantly deposited along the path of neutrophils migrating through the 3-dimensional fibrin matrix in vitro. Neutrophil-mediated CEP formation was markedly inhibited by the myeloperoxidase inhibitor, 4-ABH, and significantly reduced in myeloperoxidase-deficient mice. On macrophages, CEP adducts were recognized by cell adhesion receptors, integrin αMβ2 and αDβ2. Macrophage migration through CEP-fibrin gel was dramatically augmented when compared with fibrin alone, and was reduced by β2-integrin deficiency. Thus, neutrophil-mediated oxidation of abundant polyunsaturated fatty acids leads to the transformation of existing proteins into stronger adhesive ligands for αMβ2- and αDβ2-dependent macrophage migration. The presence of a carboxyl group rather than a pyrrole moiety on these adducts, resembling characteristics of bacterial and/or immobilized ligands, is critical for recognition by macrophages. Therefore, specific oxidation-dependent modification of extracellular matrix, aided by neutrophils, promotes subsequent αMβ2- and αDβ2-mediated migration/retention of macrophages during inflammation.
Introduction
Understanding the mechanism of leukocyte migration is essential for the treatment of chronic inflammation, which is a major factor contributing to many devastating diseases, including arthritis, diabetes, obesity, and atherosclerosis.1-3 Neutrophil recruitment is the first wave of immune response directed to fight inflammation,4 primarily by secreting peroxidases. Generation of excess reactive oxygen and nitrogen species facilitate a speedy inactivation of pathogens while releasing chemotactic signals to promote a second wave of immune response, monocyte/macrophage migration.5,6 Arriving macrophages play a central role in the resolution of acute inflammation, essentially by removing the debris and promoting tissue healing. However, excessive or uncontrolled macrophage accumulation contributes to chronic inflammation and a number of pathologies.7
To date, the mechanisms controlling the transition between the first and second wave of inflammation are not fully understood. Although chemokine gradients seem to be critical for macrophage migration, the importance of adhesive receptors and their respective ligands remains questionable. Macrophage receptors, integrins, are the major players in adhesion-mediated migration. Prominent among the leukocyte adhesion receptors are the 4 members of the integrin β2 subfamily: αLβ2 (CD11a/CD18, LFA-1), αMβ2 (CD11b/CD18, Mac-1), αXβ2 (CD11c/CD18, p150, 95), and αDβ2 (CD11d/CD18).8 Although αX is an important marker of pro-inflammatory macrophage activation, its expression on monocytes/macrophages is low, which reduces the contribution of αX to macrophage migration.9 In contrast, αMβ2 is a major β2 integrin on macrophages and αDβ2 is upregulated during pro-inflammatory macrophage activation.10 In our previous studies, we found that αMβ2 and αDβ2 are highly homologous multiligand receptors that share common ligands11,12 and participate in macrophage migration and retention at the site of inflammation.10 A typical extracellular matrix (ECM) has a limited availability of ligands for β2 integrins. The types of adhesive ligands available to mediate inflammation-specific and directed macrophage migration remain to be determined.
One of the possible mechanisms of directed macrophage migration is the modification of existing ECM during inflammation. Oxidation of polyunsaturated fatty acids (PUFA) by reactive oxygen species produced in inflamed tissues might generate protein modifications, which, in turn, might provide macrophages with inflammation-specific ligands. As oxidation substrates, PUFAs are readily available as a part of cellular membranes as well as from dietary sources, and their products were shown to exhibit a wide spectrum of biological activities.13,14 Despite widely advertised opinion regarding the beneficial role of 3-PUFA (particularly docosahexaenoate [DHA]) for overall health, current results of clinical trials are questioning its protective role for the cardiovascular system.15-17 Apparently, DHA-derived products generated in vivo may have effects distinct from DHA itself.
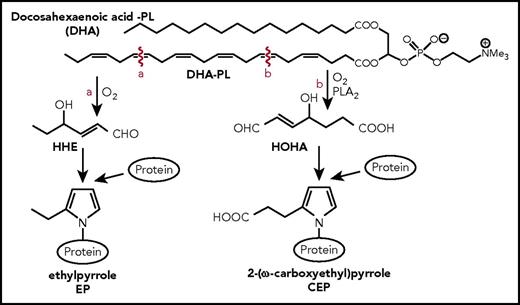
2-(ω-Carboxyethyl)pyrrole (CEP) is formed through adduction of the end products of DHA oxidation with the ε-amino groups of protein lysyl residues18,19 (Figure 1; supplemental Figure 1, available on the Blood Web site). To develop tools for testing CEP function, ω-carboxyethylpyrrole–modified proteins were synthetized using Paal-Knorr reactions of γ-dicarbonyl compounds with the ε-amino group of lysyl residues of proteins.20 γ-Dicarbonyl compounds was used to prepare CEP-modified keyhole limpet hemocyanin (CEP-KLH), bovine serum albumin (CEP-BSA), and human serum albumin). Using these proteins, highly specific monoclonal and polyclonal antibodies against CEP were generated and tested for specificity.18 Notably, a structurally similar protein modification, ethylpyrrole (EP), is generated through the alternative oxidative cleavage of DHA (Figure 1). Compared with CEP, this modification lacks a carboxyl group, which makes it an excellent control for CEP functional studies.21
Schematic representation CEP and EP formation. PLA2-catalyzed hydrolysis of DHA generates HOHA, which, in turn, produces CEP–protein derivatives through condensation with the primary amino groups of protein lysyl residues, as was described previously.18 A structurally similar protein modification, EP, is generated through the alternative oxidative cleavage of DHA to give 4-hydroxyhex-2-enal followed by condensation of 4-hydroxyhex-2-enal with the ε-amino group of lysyl residues. Compared with CEP, this modification lacks a carboxyl group. HHE, 4-hydroxyhexenal; HOHA, 4-hydroxy-7-oxo-hept-5-eonate.
Schematic representation CEP and EP formation. PLA2-catalyzed hydrolysis of DHA generates HOHA, which, in turn, produces CEP–protein derivatives through condensation with the primary amino groups of protein lysyl residues, as was described previously.18 A structurally similar protein modification, EP, is generated through the alternative oxidative cleavage of DHA to give 4-hydroxyhex-2-enal followed by condensation of 4-hydroxyhex-2-enal with the ε-amino group of lysyl residues. Compared with CEP, this modification lacks a carboxyl group. HHE, 4-hydroxyhexenal; HOHA, 4-hydroxy-7-oxo-hept-5-eonate.
CEP generation was reported to contribute to a number of inflammation-associated diseases, including macular degeneration, hyperlipidemia, atherosclerosis, thrombosis, and tumor progression.21,22 However, the pro-inflammatory mechanism of CEP function is not clear. Accumulation of CEP in damaged tissue and induction of pro-inflammatory cytokines from macrophages in response to CEP represents the data that may explain the contribution of CEP to the augmentation of inflammatory responses.23-25 Our investigation was outlined to seek a possible link between CEP and macrophage migration/accumulation at the site of inflammation.
Materials and methods
Additional information is available in the supplemental Materials and methods.
Mice
Wild-type (WT; C57BL/6J) mice, β2-deficient mice (B6.129S7-Itgb2tm2Bay/J), and myeloperoxidase (MPO)-deficient mice (B6.129X1-Mpotm1Lus/J) were purchased from Jackson Laboratory (Bar Harbor, ME). All procedures were performed according to animal protocols approved by the Cleveland Clinic and East Tennessee State University Institutional Animal Care and Use Committee.
Cell adhesion and 2D migration
Neutrophil and macrophage 3D migration in fibrin matrix
Three-dimensional (3D) migration assay was performed as described in our previous paper.10 Briefly, neutrophils or macrophages were labeled with PKH26 red or PKH67 green fluorescent dyes. Labeled leukocytes migrated through the custom-made fibrin matrix prepared in Transwell inserts for 24 (neutrophils) or 48 (macrophages) hours at 37°C in 5% CO2. 100 nM N-formylmethionyl-leucyl-phenylalanine (FMLP), or 30 nM monocyte chemoattractant protein-1 (MCP-1) were added on the top of the gel to initiate the migration. Migrating cells were detected by Leica Confocal microscope (Leica-TCS SP8) and the results were analyzed by IMARIS 8.0 software.
Isolation of recombinant αD, αM, and αL I-domains and real-time protein–protein interaction
Constructs for αD I-domains, αM I-domains, and the αL I-domain in active and nonactive conformations were generated and recombinant proteins were isolated as described in our previous papers.12,26 Real-time protein–protein interactions were analyzed using a Biacore3000 instrument (Biacore, Uppsala, Sweden) as described previously.12,21
Results
CEP is involved in macrophage accumulation in the peritoneal cavity
Numerous data demonstrate the involvement of CEP in the inflammatory process.21,23-25 We tested CEP and EP accumulation during acute inflammation in the peritoneal tissue after thioglycollate-induced peritoneal inflammation. Normal tissue is characterized by strong deposition of EP, but was devoid of CEP completely. However, induction of inflammation led to the marked accumulation of CEP in the peritoneal wall (supplemental Figure 2A) that significantly overlaps with macrophage staining after 72 hours (Figure 2 A-B), which indicates a link between CEP and macrophage accumulation during peritoneal inflammation. Anti-CEP monoclonal antibody was injected into the peritoneal cavity of WT mice 30 minutes before and 24 hours after injection of thioglycollate. We found that thioglycollate-induced accumulation of macrophages in the peritoneal cavity after 72 hours was dramatically reduced in the presence of anti-CEP antibody, whereas neutrophil accumulation after either 6 or 18 hours was not affected (Figure 2C; supplemental Figure 2B). Notably, anti-CEP antibody did not affect cell survival according to cell toxicity assay (Dojindo Molecular Technologies, Rockville, MD) (supplemental Figure 2C).
Deposition of EP and CEP in normal and inflamed peritoneal tissues. Peritoneal tissues were isolated from mice at 72 hours after thioglycollate-induced inflammation (A, lower panels) or from nontreated mice as a control (A, upper panels). Immunofluorescent staining demonstrates EP or CEP (green fluorescence) and CD68 (red fluorescence). Magnifications ×200. (B) CEP and EP staining in noninflamed (green bars) and inflamed (blue bars) tissues were analyzed using Fiji software. (C) Neutrophil and macrophage accumulation in the peritoneal cavity during thioglycollate-induced peritoneal inflammation after anti-CEP mAb treatment. Mice were injected twice with anti-CEP mAb or IgM control (30 minutes before and 24 hours after injection of 1 mL of 4% thioglycollate). Neutrophils were isolated at 18 hours and macrophages were isolated at 72 hours after thioglycollate injection. Statistical analysis was performed using Student t test. (D) Immunoprecipitation with anti-CEP mAb. Mouse peritoneal exudate was isolated at 72 hours after injection of thioglycollate and incubated with anti-CEP antibody. Antibody-bound fraction was separated by Laemmli SDS gradient electrophoresis (4% -15%). Fg was detected by mass spectrometry in the major bands isolated by immunoprecipitation with anti-CEP mAb. (E) Western blot analysis with anti-CEP pAb after immunoprecipitation with anti-Fg mAb. The same peritoneal exudate was immunoprecipitated with anti-Fg polyclonal antibody and the isolated fraction was analyzed by western blot with anti-CEP polyclonal antibody. Fg, fibrinogen; IP, immunoprecipitation; mAb, monoclonal antibody; n/s, not significant; pAb, polyclonal antibody; WB, western blotting.
Deposition of EP and CEP in normal and inflamed peritoneal tissues. Peritoneal tissues were isolated from mice at 72 hours after thioglycollate-induced inflammation (A, lower panels) or from nontreated mice as a control (A, upper panels). Immunofluorescent staining demonstrates EP or CEP (green fluorescence) and CD68 (red fluorescence). Magnifications ×200. (B) CEP and EP staining in noninflamed (green bars) and inflamed (blue bars) tissues were analyzed using Fiji software. (C) Neutrophil and macrophage accumulation in the peritoneal cavity during thioglycollate-induced peritoneal inflammation after anti-CEP mAb treatment. Mice were injected twice with anti-CEP mAb or IgM control (30 minutes before and 24 hours after injection of 1 mL of 4% thioglycollate). Neutrophils were isolated at 18 hours and macrophages were isolated at 72 hours after thioglycollate injection. Statistical analysis was performed using Student t test. (D) Immunoprecipitation with anti-CEP mAb. Mouse peritoneal exudate was isolated at 72 hours after injection of thioglycollate and incubated with anti-CEP antibody. Antibody-bound fraction was separated by Laemmli SDS gradient electrophoresis (4% -15%). Fg was detected by mass spectrometry in the major bands isolated by immunoprecipitation with anti-CEP mAb. (E) Western blot analysis with anti-CEP pAb after immunoprecipitation with anti-Fg mAb. The same peritoneal exudate was immunoprecipitated with anti-Fg polyclonal antibody and the isolated fraction was analyzed by western blot with anti-CEP polyclonal antibody. Fg, fibrinogen; IP, immunoprecipitation; mAb, monoclonal antibody; n/s, not significant; pAb, polyclonal antibody; WB, western blotting.
To detect CEP-modified ligands for macrophages within the peritoneal cavity, we collected mouse peritoneal exudates at 72 hours after injection of thioglycollate and performed immunoprecipitation with anti-CEP antibody. The sodium dodecyl sulfate (SDS)-electrophoresis demonstrated 2 bands with the molecular weights ∼70 and ∼49 to 51 kDa (reduced samples) (Figure 2D). Using mass spectrometry we identified 2 major proteins in these bands: fibrinogen and albumin. Serum albumin (69 kDa; 35 peptides, covering 51.3% sequences) and the α-chain of fibrinogen (67 kDa; 8 peptides, covering 15.1% sequences) were detected in the upper band.27 ɤ-Chains of fibrinogen (47 kDa; 2 peptides, 4.8% of sequences) were identified in the lower band (Figure 2D). To confirm the presence of CEP-modified fibrinogen, we performed immunoprecipitation of mouse peritoneal exudates with anti-fibrinogen antibody. A subsequent western blot with anti-CEP antibody revealed a band that corresponds to the molecular weight of the fibrinogen α-chain (Figure 2E). The bands, which correspond to the β and ɣ chains, are masked by the heavy chains of immunoglobulin G. Therefore, peritoneal inflammation led to the formation of CEP-modified fibrinogen and albumin, which are major plasma proteins that leak into the tissue during inflammation.
Neutrophil migration in vitro generates CEP-modified proteins
Neutrophil activation during inflammation promotes the release of MPO and other enzymes leading to generation of reactive oxygen and nitrogen species, causing oxidation of polyunsaturated lipids.28 Polyunsaturated phospholipids are abundant in the membranes of cells, particularly neutrophils.29 We explored the possibility that neutrophil accumulation and activation may lead to generation of CEP-modified proteins within ECM. To test this hypothesis in vitro, a 3-dimensional fibrin matrix was generated in a Boyden chamber. Freshly isolated neutrophils were stained with green fluorescent dye and stimulated by an FMLP gradient to migrate through the fibrin matrix. After overnight migration, the fibrin matrix was stained with anti-CEP antibody (red fluorescence). We detected a dramatic increase in CEP deposition following neutrophil migration (Figure 3A-B). In parallel samples, fibrin gels were digested with plasmin. The generated protein fragments were tested by western blot using an anti-CEP antibody. A strong CEP signal was detected in western blot samples after neutrophil migration. The band at ∼180 kDa (nonreduced samples) corresponds to the molecular weight of the DD-fragment of fibrin, the major product of fibrin degradation by plasmin. The sequences from the β- and ɣ-chains of fibrin were detected in this band by mass spectrometry (β-chain: 10 peptides, 31% sequence coverage; ɣ-chain: 7 peptides, 21.74% sequence coverage). The band at ∼69 kDa corresponds to the molecular weight of bovine albumin, which was also verified by mass spectrometry (32 peptides, 59.31% sequence coverage). Bovine albumin (1% fetal bovine serum) was incorporated in the fibrin matrix for neutrophil support. Surprisingly, the second major product of fibrin degradation, the E-fragment (50 kDa) was not detected by anti-CEP antibodies, which indicates selectivity for protein modification by CEP. Notably, the control fibrin gel had only background staining, which is most likely because of the low level of preexisting modifications present in isolated fibrinogen (Figure 3 A,C). The contribution of neutrophils and macrophages to CEP formation was evaluated using neutrophil depleted (Ly6G antibody) and macrophage depleted (Clodronate-liposome) models. Neutrophil depletion dramatically reduced the formation of CEP in the peritoneal cavity of mice after thioglycollate induced peritoneal inflammation, whereas macrophage depletion had no significant effect. These data confirmed our in vitro results and emphasize the role of neutrophils in CEP generation (supplemental Figure 3).
Neutrophil migration generates CEP formation. (A) Neutrophils were isolated from human blood and labeled with green fluorescent dye PKH67. Fibrin gel supplemented with 1% fetal bovine serum and 100 ng/mL LPS was polymerized in a Boyden chamber. Labeled neutrophils were plated on top of the fibrin gel in the lower chamber. Migration was initiated by adding 100 nM FMLP to the upper chamber. After 18 hours, the gel was fixed and stained with anti-CEP antibody (red). Control fibrin gel was incubated without neutrophils. (B) Quantification of intensity of CEP staining of 15 fields in 3 samples was detected by confocal microscopy. (C) Separated samples after migration were digested using plasmin and analyzed by SDS-electrophoresis and western blot using anti-CEP antibody. DD- fragment of fibrin (180 kDa). E-fragment of fibrin (55 kDa). (D) CEP formation was inhibited in the presence of MPO inhibitor, 4-ABH, but was not affected by eosinophil peroxidase inhibitor, resorcinol. (E) Accumulation of CEP but not EP is aided by MPO. Wounded tissues were collected from WT and MPO-deficient mice and stained for EP and CEP. *P < .05; **P < .01. LPS, lipopolysaccharide.
Neutrophil migration generates CEP formation. (A) Neutrophils were isolated from human blood and labeled with green fluorescent dye PKH67. Fibrin gel supplemented with 1% fetal bovine serum and 100 ng/mL LPS was polymerized in a Boyden chamber. Labeled neutrophils were plated on top of the fibrin gel in the lower chamber. Migration was initiated by adding 100 nM FMLP to the upper chamber. After 18 hours, the gel was fixed and stained with anti-CEP antibody (red). Control fibrin gel was incubated without neutrophils. (B) Quantification of intensity of CEP staining of 15 fields in 3 samples was detected by confocal microscopy. (C) Separated samples after migration were digested using plasmin and analyzed by SDS-electrophoresis and western blot using anti-CEP antibody. DD- fragment of fibrin (180 kDa). E-fragment of fibrin (55 kDa). (D) CEP formation was inhibited in the presence of MPO inhibitor, 4-ABH, but was not affected by eosinophil peroxidase inhibitor, resorcinol. (E) Accumulation of CEP but not EP is aided by MPO. Wounded tissues were collected from WT and MPO-deficient mice and stained for EP and CEP. *P < .05; **P < .01. LPS, lipopolysaccharide.
Because MPO is a major peroxidase enzyme secreted by neutrophils especially upon their activation, we tested its role in CEP generation. Activation of human neutrophils with interleukin-1β (IL-1β; 200 ng/mL) or lipopolysaccharide (100 ng/mL) increased CEP levels in the media by more than twofold, thereby confirming that neutrophil activation promotes CEP generation (Figure 3D). The treatment of neutrophils with the MPO inhibitor, 4-ABH, dramatically reduced the CEP level. 4-ABH was used at a concentration known to have no effect on catalase or glutathione peroxidase activities or on the production of superoxide by neutrophils.30 At the same time, resorcinol at a concentration of 10 µM had no effect on CEP production. It has been shown that at this concentration resorcinol inhibits eosinophil peroxidase but has low effect on MPO.31 In contrast to CEP, neither 4-ABH nor resorcinol inhibited the production of EP by neutrophils (supplemental Figure 4). Consistent with the main role for MPO in CEP generation, CEP levels in wounds of MPO-deficient mice were reduced by 50% as compared with WT controls (Figure 3E). To assess whether MPO is able to directly contribute to CEP–protein adduct formation, human fibrinogen was incubated with active recombinant MPO and DHA.32 Resulting CEP formation was quantified by enzyme-linked immunosorbent assay (supplemental Figure 5A). As anticipated,33 the formation of CEP–protein adducts required the presence of all 3 main components: DHA (as a lipid substrate), MPO (as a source of oxidation), and a protein (eg, fibrinogen, as a source of lysines). Incubation of fibrinogen with MPO or DHA alone was not sufficient for CEP generation. The presence of CEP adducts on fibrinogen was confirmed by western blot with monoclonal anti-CEP antibody (supplemental Figure 5B). These results clearly demonstrate that MPO-mediated DHA oxidation is one of the main mechanisms for CEP generation. Based on the results with MPO-deficient mice, MPO seems to be a clear source of CEP generation in vivo; however, it appears that other oxidative enzymes might participate in this process.
Macrophages adhere to CEP in an integrin β2–dependent manner
During macrophage migration, CEP may serve as a chemoattractant, an activator of adhesive receptors, or a direct ligand for adhesive receptors. First, using a 2D chemotactic assay, we found that neither CEP nor EP was able to stimulate macrophage chemotaxis. MCP-1 was used as a positive control, whereas BSA was used as a negative control (supplemental Figure 6A). Next, we found that preincubation with CEP did not increase macrophage adhesion to immobilized fibronectin34 (ligand to different β1, β2, and β3 integrins), which eliminates a potential effect of CEP on integrin activation (supplemental Figure 6B).
Based on these results, we hypothesized that CEP may serve as a direct ligand for macrophage adhesion and migration. Adhesion assay revealed strong binding of peritoneal macrophages to CEP-modified BSA and CEP-modified KLH, whereas adhesion to EP-modified BSA or BSA itself was not detected (Figure 4A). Because leukocyte integrins are the major migratory receptors on the macrophage surface, we evaluated a contribution of 2 macrophage integrin subfamilies β1 and β2 on CEP-mediated adhesion. The adhesion to CEP was significantly inhibited by anti-β2, but not by anti-β1 antibodies (Figure 4B). These data suggest that CEP may serve as a ligand for β2-integrins.
Macrophages adhere to the CEP-modified proteins via β2integrins. (A-B) Peritoneal macrophages. Ninety-six-well plates were coated with different ligands for 3 hours at 37°C. (A) Fluorescently labeled macrophages were added to the wells and cell adhesion was determined after 30 minutes in a fluorescence plate reader. (B) Some samples were preincubated with anti-β2 and anti-β1 blocking antibodies before the adhesion assay.**P < .01. (C-F) HEK 293–transfected cells. (C) αMβ2, αDβ2, and αLβ2-HEK 293 transfected cells were generated as described in the “Materials and methods” section and tested by flow cytometry analysis. The mock-transfected cells are shown only with anti-αM mAb. A similar result was obtained with anti-αL and anti-αD antibodies. Ninety-six-well plates were coated with CEP (D) or different ligands (E-F) for 3 hours at 37°C. αMβ2, αDβ2, αLβ2, or mock-transfected cells were labeled with 10 µM Calcein AM. (E-F) For some experiments, cells were preincubated with anti-integrin blocking antibodies. In separate wells, immobilized CEP-BSA was preincubated with anti-CEP mAb. After incubation, cells were added to the wells and cell adhesion was determined after 30 minutes in a fluorescence plate reader. Statistical analyses were performed using Student t test. Fn, fibronectin.
Macrophages adhere to the CEP-modified proteins via β2integrins. (A-B) Peritoneal macrophages. Ninety-six-well plates were coated with different ligands for 3 hours at 37°C. (A) Fluorescently labeled macrophages were added to the wells and cell adhesion was determined after 30 minutes in a fluorescence plate reader. (B) Some samples were preincubated with anti-β2 and anti-β1 blocking antibodies before the adhesion assay.**P < .01. (C-F) HEK 293–transfected cells. (C) αMβ2, αDβ2, and αLβ2-HEK 293 transfected cells were generated as described in the “Materials and methods” section and tested by flow cytometry analysis. The mock-transfected cells are shown only with anti-αM mAb. A similar result was obtained with anti-αL and anti-αD antibodies. Ninety-six-well plates were coated with CEP (D) or different ligands (E-F) for 3 hours at 37°C. αMβ2, αDβ2, αLβ2, or mock-transfected cells were labeled with 10 µM Calcein AM. (E-F) For some experiments, cells were preincubated with anti-integrin blocking antibodies. In separate wells, immobilized CEP-BSA was preincubated with anti-CEP mAb. After incubation, cells were added to the wells and cell adhesion was determined after 30 minutes in a fluorescence plate reader. Statistical analyses were performed using Student t test. Fn, fibronectin.
αMβ2 and αDβ2, but not αLβ2-transfected cells, adhere to CEP
The subfamily of β2 integrins consists of 4 members, αMβ2, αDβ2, αLβ2, and αXβ2. Although αMβ2, αDβ2, and αLβ2 demonstrate strong expression on macrophages, the level of integrin αXβ2 is low, which reduces its potential role in integrin-mediated adhesion and migration. To further confirm the role of β2 integrins, we used αMβ2-, αDβ2-, and αLβ2-transfected HEK293 cells previously generated in our laboratory (Figure 4C).11,12 We tested their ability to bind CEP-BSA and found that αDβ2- and αMβ2-transfected, but not αLβ2-transfected or control mock-transfected cells, strongly adhered to CEP (Figure 4D). In contrast, adhesion to BSA or EP was not detected for αDβ2- and αMβ2-transfected cells (Figure 4E-F). HEK 293 cells express endogenous β1 integrins including α1β1, α2β1, α4β1, and α5β1; therefore, the lack of adhesion of mock-transfected HEK293 cells to CEP confirmed integrin β2 specificity for CEP.
The adhesion of αDβ2- and αMβ2-transfected cells was significantly inhibited by anti-CEP, anti-β2, and anti-αD (or anti-αM) antibodies, but not by anti-β1 antibody (Figure 4E-F). In addition, we demonstrated that preincubation of αMβ2 and αDβ2 cells with CEP in solution decreased the binding of blocking anti-αM and anti-αD antibodies more than twofold (supplemental Figure 7). These data prove the hypothesis that integrin αMβ2 and αDβ2 are receptors for CEP-modified proteins.
Isolated recombinant αM and αD I-domains interact with CEP
It has been shown that the ligand binding sites for most ligands of αMβ2 and αDβ2 integrins reside within the I-domain, the independently folded domain that sits on the top of the extracellular part of the integrin.35 To verify our adhesion results, we expressed recombinant I-domains, αD in active and inactive conformation, αM in active and inactive conformation, and αL in active conformation (Figure 5A) and tested their direct binding to the CEP-BSA using surface plasmon resonance. The binding to EP-BSA was evaluated as a control. The different concentrations of αD and αM I-domains flowed over the immobilized CEP or EP. We found that αD and αM I-domains in active conformation demonstrated concentration-dependent binding to CEP (Figure 5D-E). In contrast, binding to EP was not detected (Figure 5B-C). The distribution coefficient for CEP–I-domain interactions were calculated using BIAevaluation software (GE Healthcare) and correspond to 2.1 × 10−6 for αM and 1.81 × 10−7 for αD. This binding was activation dependent because αM and αD I-domains in nonactive conformation did not bind to CEP (Figure 5F-G). The specificity of binding was confirmed with blocking anti-αM antibodies (supplemental Figure 8). Active αL I-domain did not interact with immobilized CEP (Figure 5G). These data verified our results obtained in adhesion assay with αMβ2, αDβ2, and αLβ2 transfected cells.
Isolated αM and αD I-domains directly bind to CEP in surface plasmon resonance assays. (A) SDS-PAGE of generated I-domains. The I-domains αL active (G127-Y307), αD nonactive (P128-S323), αD active (D122-K314), αM nonactive (Q119-E333), and αM active (E123-K315) were isolated from soluble fractions of Escherichia coli lysates, purified using affinity chromatography, and their purity assessed by SDS-PAGE on 4% to 20% gradient gel under reducing conditions followed by staining with Coomassie Blue. (B-G) Representative profiles of the SPR responses for αM (B) and αD (C) binding to the immobilized CEP-BSA and EP-BSA. Binding of αM I-domain (D) and αD I-domain (E) in active conformation (concentrations ranging from 16 to 500 nM) to CEP-BSA coupled to the CM5 chip. (F) αM I-domain in nonactive conformation has significantly reduced binding to CEP-BSA. (G) αD I-domain in nonactive conformation and αL I-domain in active conformation demonstrate very low binding to CEP-BSA. PAGE, polyacrylamide gel electrophoresis; SPR, surface plasmon resonance.
Isolated αM and αD I-domains directly bind to CEP in surface plasmon resonance assays. (A) SDS-PAGE of generated I-domains. The I-domains αL active (G127-Y307), αD nonactive (P128-S323), αD active (D122-K314), αM nonactive (Q119-E333), and αM active (E123-K315) were isolated from soluble fractions of Escherichia coli lysates, purified using affinity chromatography, and their purity assessed by SDS-PAGE on 4% to 20% gradient gel under reducing conditions followed by staining with Coomassie Blue. (B-G) Representative profiles of the SPR responses for αM (B) and αD (C) binding to the immobilized CEP-BSA and EP-BSA. Binding of αM I-domain (D) and αD I-domain (E) in active conformation (concentrations ranging from 16 to 500 nM) to CEP-BSA coupled to the CM5 chip. (F) αM I-domain in nonactive conformation has significantly reduced binding to CEP-BSA. (G) αD I-domain in nonactive conformation and αL I-domain in active conformation demonstrate very low binding to CEP-BSA. PAGE, polyacrylamide gel electrophoresis; SPR, surface plasmon resonance.
CEP stimulates migration of macrophages via β2 integrin
To characterize CEP as a migratory substrate for αMβ2 and αDβ2 integrins, we first demonstrated that β2 deficiency significantly reduced macrophage adhesion to CEP (Figure 6A). Then, we compared the migration of WT and β2-deficient macrophages within 3D CEP-enriched fibrin matrix. WT cells and β2-deficient cells were labeled with green fluorescent dye PKH67 and red fluorescent dye PKH26, respectively. Macrophages were mixed at a ratio of 1:1 (Figure 6B), placed on the bottom of a fibrin gel in a Boyden chamber, and then migration against gravity was initiated by MCP-1 added to the top surface of the gel. The migration was evaluated after 48 hours (Figure 6C-D) and revealed that β2 deficiency dramatically reduced the 3D migration of macrophages into the CEP-enriched matrix (Figure 6E). To exclude the contribution of a particular dye, the experiment was repeated with the opposite labeling conditions and revealed a similar result (supplemental Figure 9). To demonstrate that the difference between WT control and β2-deficient macrophages was due to mesenchymal rather than amoeboid migration, this experiment was performed in the presence of ROCK inhibitor (Y-27632), known to block the amoeboid (adhesion-independent) component of cell migration. The presence of ROCK inhibitor did not alter the pattern of migration and the difference between WT control and β2-deficient macrophages remained. This confirms that macrophage migration in 3D CEP-enriched matrix was integrin-dependent, or mesenchymal type (supplemental Figure 10). The quality of ROCK inhibitor was verified using M2-activated macrophages that strongly depend on amoeboid motility (data not shown).
CEP-dependent macrophage migration in a 3D matrix. (A) Thioglycollate-induced peritoneal macrophages were isolated from WT or β2−/− mice and their adhesion to CEP was evaluated as described for Figure 4. (Bi) Isolated WT and β2−/− macrophages were labeled with green (WT) or red (β2−/−) fluorescent dyes. Cells were mixed in equal number and the similar amounts of cells were verified by cytospin of mixed cells. Bar represents 400 μm. (Bii) The cell number was calculated by Image Analysis Software (EVOS, Thermo Fisher) using 5 random fields. (C-E) Thrombin-treated fibrinogen forms a 3D polymerized gel in a Boyden chamber. (Ci) Labeled cells were plated on 3D polymerized fibrin in transwell inserts. Migration of macrophages was stimulated by 30 nM MCP-1 added to the top of the gel. (Cii and D) After 48 hours, migrating cells were detected by a Leica Confocal microscope The first 30 μm of the gel from the starting point (where many nonmigrated cells reside) is not shown to reduce a gradient of brightness intensity for the sample. (E) The results were analyzed by IMARIS 8.0 software and plotted. Statistical analyses were performed using Student paired t tests (n = 4 samples per group). Bar represents 500 μm.
CEP-dependent macrophage migration in a 3D matrix. (A) Thioglycollate-induced peritoneal macrophages were isolated from WT or β2−/− mice and their adhesion to CEP was evaluated as described for Figure 4. (Bi) Isolated WT and β2−/− macrophages were labeled with green (WT) or red (β2−/−) fluorescent dyes. Cells were mixed in equal number and the similar amounts of cells were verified by cytospin of mixed cells. Bar represents 400 μm. (Bii) The cell number was calculated by Image Analysis Software (EVOS, Thermo Fisher) using 5 random fields. (C-E) Thrombin-treated fibrinogen forms a 3D polymerized gel in a Boyden chamber. (Ci) Labeled cells were plated on 3D polymerized fibrin in transwell inserts. Migration of macrophages was stimulated by 30 nM MCP-1 added to the top of the gel. (Cii and D) After 48 hours, migrating cells were detected by a Leica Confocal microscope The first 30 μm of the gel from the starting point (where many nonmigrated cells reside) is not shown to reduce a gradient of brightness intensity for the sample. (E) The results were analyzed by IMARIS 8.0 software and plotted. Statistical analyses were performed using Student paired t tests (n = 4 samples per group). Bar represents 500 μm.
To rule out the potential interplay between WT and β2−/− cells in 3D gel, we tested the migration of individual subsets of macrophages in CEP- and EP-enriched fibrin matrices (Figure 7A-B). Similar to the results of the adhesion experiments (Figure 4A), the migration of WT macrophages involving CEP was substantially stronger in comparison with EP. In contrast, the migration of β2−/−-deficient macrophages was similar between CEP and EP (Figure 7B), which clearly indicates the critical role of β2 integrins, primarily αMβ2 and αDβ2 (Figures 4 and 5), in CEP-mediated migration.
CEP supplemented in 3D fibrin matrix increases macrophage migration, but not neutrophil migration. Thrombin-treated fibrinogen forms a 3D polymerized gel in a Boyden chamber. Thioglycollate-induced WT (A) or β2−/− (B) peritoneal macrophages were labeled with PKH67 green fluorescent dye and plated on the gel. Macrophage migration was stimulated with 30 nM MCP-1. A total of 9 µM EP (left) or CEP (right) was incorporated in the gel during polymerization. Results were evaluated in 4 to 6 samples per group (9 field of view per sample), analyzed by IMARIS 8.0 software, and plotted. (C) Neutrophils were labeled with PKH26 red fluorescent dye and plated on fibrin matrix with incorporated CEP or EP. The migration was detected after 24 hours as described for macrophages. The first 30 μm of the gel from the starting point (where many nonmigrated cells reside) is not shown to reduce a gradient of brightness intensity for the sample. Statistical analyses were performed using Student paired t tests (n = 4 per group). Bar represents 500 μm.
CEP supplemented in 3D fibrin matrix increases macrophage migration, but not neutrophil migration. Thrombin-treated fibrinogen forms a 3D polymerized gel in a Boyden chamber. Thioglycollate-induced WT (A) or β2−/− (B) peritoneal macrophages were labeled with PKH67 green fluorescent dye and plated on the gel. Macrophage migration was stimulated with 30 nM MCP-1. A total of 9 µM EP (left) or CEP (right) was incorporated in the gel during polymerization. Results were evaluated in 4 to 6 samples per group (9 field of view per sample), analyzed by IMARIS 8.0 software, and plotted. (C) Neutrophils were labeled with PKH26 red fluorescent dye and plated on fibrin matrix with incorporated CEP or EP. The migration was detected after 24 hours as described for macrophages. The first 30 μm of the gel from the starting point (where many nonmigrated cells reside) is not shown to reduce a gradient of brightness intensity for the sample. Statistical analyses were performed using Student paired t tests (n = 4 per group). Bar represents 500 μm.
Because neutrophils also express αMβ2 and αDβ2 integrins, neutrophil migration might also depend on the presence of CEP within the matrix. We evaluated the migration of human neutrophils within CEP-enriched fibrin gel; however, no additional effects of CEP on neutrophil migration were detected (Figure 7C). These results correspond to the published data showing that, in contrast to macrophages, neutrophil 3D migration is exclusively mediated by the amoeboid mode.36
Discussion
In the present study, we demonstrated that recruited neutrophils secrete reactive oxygen species that modify existing components of ECM by CEP adducts, which in turn serve as adhesive and migratory ligands for macrophage integrins. The following conclusions are drawn from this study. (1) Although CEP is not present in healthy tissues, levels are dramatically increased at the sites of inflammation. (2) Neutrophil activation and migration through ECM results in the generation of CEP-modified proteins. (3) CEP-modified proteins promote macrophage adhesion and migration. (4) Inhibition of CEP prevents macrophage recruitment. (5) Integrins αMβ2 and αDβ2 are specific macrophage receptors for CEP-mediated adhesion and migration. Thus, CEP is a natural inflammatory product generated during the first phase of inflammation by recruited neutrophils, facilitating the second wave of inflammation, namely, the recruitment of macrophages. By means of oxidation, neutrophils seem to “pave the road” for future macrophage invasion by modifying ECM with CEP.
CEP was initially described in the retina of patients with age-related macular degeneration.18 Later, however, the link between inflammation and CEP was demonstrated in several pathologies associated with chronic inflammation including atherosclerosis, tumor progression, aging, and others.22,26,37-39 Several groups reported the secretion of pro-inflammatory mediators from macrophages after engagement with CEP-modified proteins.21,23-25 In agreement with this, CEP induced M1 macrophage polarization.40 Therefore, CEP function is closely related to the development of inflammation.
Previously, several receptors were detected for CEP on the surface of macrophages, including TLR2, CD36, TLR1, and TLR6.21,38 Although these receptors contribute to macrophage activation and reprogramming by CEP, they do not directly mediate macrophage migration. Our data demonstrated that CEP is not able to serve as a chemoattractant for macrophages or as an integrin activation agonist. These results suggest that macrophages use another receptor for CEP that can mediate migration.
A subfamily of β2 integrins are the major adhesive receptors on the surface of macrophages. Since αLβ2 interacts only with cell counterreceptors and the expression of αXβ2 on macrophages is low,9 2 other members of the subfamily, αMβ2 and αDβ2, are the best candidates for macrophage migration and retention in the extracellular matrix. αDβ2 and αMβ2 recognize a wide range of ligands, including fibronectin, thrombospondin, and vitronectin.11,12 CEP modifications seem to generate the new inflammation-specific substrate for αMβ2- and αDβ2-mediated macrophage migration or retention. Importantly, even known β2 integrin ligands such as fibrinogen can be “improved” by CEP modification. Inflammation promotes the leakage of fibrinogen from blood to the inflamed tissue. Importantly, although the affinity of soluble fibrinogen for β2 integrins is low, it increases substantially upon immobilization or partial digestion because of the exposure of carboxyl groups of glutamic and aspartic acid.41,42 Acidic side chains of ligands are required for the binding to Mg2+ in the MIDAS motif within the integrin I-domain, which is critical for integrin ligand recognition.43 Likewise, the modification of fibrin with CEP (via the side-chain of lysine) reduces the positive charge on the fibrin surface and at the same time increases the number of negatively charged carboxyl groups, which are a major active element within the CEP structure. Thus, CEP adducts modify fibrinogen into the high-affinity ligand for αMβ2 and αDβ2 integrins. Indeed, we show that CEP but not EP, which modifies proteins in a similar manner but lacks a carboxyl group, is able to create an adhesive β2 ligand out of many proteins, possibly imitating bacterial ligands by exposed carboxyl groups as proposed previously. In general, the high distribution coefficient for the αM-CEP and αD-CEP interactions demonstrate a strong affinity that exceeds the affinity for the binding of αM or αD I-domains to previously identified proteins.12 This makes CEP-modified proteins a preferential ligand for the macrophages. We found that the affinity for αD-CEP interaction surpasses the affinity for αM-CEP approximately 10-fold. We suggest that this effect is mediated by a difference in the electrostatic surfaces between the αD I-domain and αM I-domain.44 Therefore, integrin αDβ2 may play a more significant physiological role in the CEP-mediated macrophage adhesion and migration. Further studies are required to clarify this question.
It is well accepted that strong integrin-mediated adhesion often antagonizes or limits migration.45,46 Because CEP-modified proteins serve as strong ligands for β2 on macrophages, CEP might serve as a cell migration signal for cells with moderate integrin expression and as a retention/arrest signal for cells with the high integrin expression. We recently demonstrated that expression of αDβ2 is upregulated on M1 macrophages in vitro and within atherosclerotic lesions. We showed that high expression of αDβ2 mediates the retention of macrophages at the site of inflammation.10 Thus, CEP-modified proteins are the likely ligands responsible for these phenomena.
Recent studies indicate that rapid migration of neutrophils is mediated in an integrin-independent manner by an amoeboid mode of motility.36 Our data correspond to these results (Figure 7C) and confirm that neutrophils do not need to use CEP as substrate for their migration. Importantly, it has been demonstrated that macrophages can use both mechanisms, amoeboid and mesenchymal migration, depending on environment and macrophage subset.47 This, again, implies the selectivity of CEP ligand specificity toward macrophages.
Macrophage accumulation serves as defensive mechanism during acute inflammation48 or wound healing,49 but has a strong pathological outcome during the development of chronic inflammation.50 Therefore, CEP generation may have pro-inflammatory as well as protective functions, depending on type of inflammation. The information obtained in our studies not only establishes the foundation for a new model of inflammation but also provides a new strategy for treatment of chronic inflammatory diseases. The advantage of CEP as a new therapeutic target resides in its unique formation in inflamed tissue. Therefore, the blocking of CEP in peripheral tissues during different chronic inflammatory diseases can prevent macrophage accumulation and further development of chronic inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
These studies were supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant DK102020 (V.P.Y.), National Institutes of Health, National Heart, Lung, and Blood Institute grants HL077213 (E.A.P.), HL126738 (E.A.P.), and HL071625 (T.V.B.), National Institutes of Health, National Eye Institute grant EY016813 (R.G.S.), National Institutes of Health grant C06RR0306551 for East Tennessee State University, and by the Canova endowment fund (T.V.B.).
Authorship
Contribution: V.P.Y. designed the research, performed the experiments, analyzed the data, and wrote the manuscript; K.C., C.L.A., D.G., K.E.B., S.S., and X.Z.W. performed the experiments and analyzed the data; R.G.S. provided 2-(ω-carboxyethyl)pyrrole– and ethylpyrrole-modified proteins, anti-CEP polyclonal antibody, and edited the manuscript; E.A.P. designed the research and analyzed the data; and T.V.B. designed the research, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valentin P. Yakubenko, Department of Biomedical Sciences, Quillen College of Medicine, East Tennessee State University, PO Box 70582, Johnson City, TN 37614; e-mail: yakubenko@etsu.edu.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal