Key Points
The outpatient BV and bendamustine regimen is highly active as first salvage therapy in relapsed/refractory HL, with manageable toxicity.
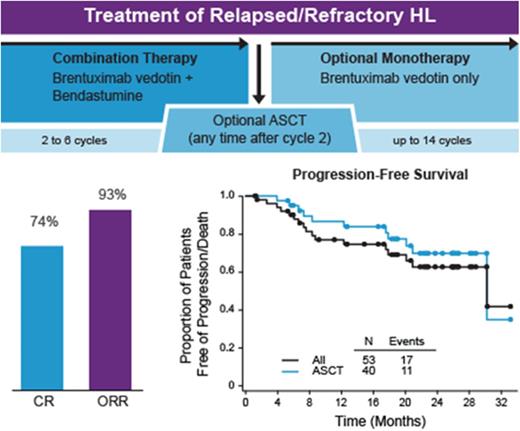
The CR rate of 73.6% exceeded those reported for standard chemotherapy regimens, and post-ASCT outcomes generally appeared excellent.
Abstract
Autologous stem cell transplantation (ASCT) is standard of care for patients with Hodgkin lymphoma (HL) who have relapsed/refractory disease after frontline chemotherapy. Achievement of complete remission (CR) with pre-ASCT salvage chemotherapy predicts favorable outcomes post-ASCT. This phase 1/2 study evaluated the combination of brentuximab vedotin (BV) plus bendamustine as a first salvage regimen in relapsed/refractory HL. A total of 55 patients (28 primary refractory and 27 relapsed) were enrolled. Patients received BV (1.8 mg/kg) on day 1 and bendamustine (90 mg/m2) on days 1 and 2 of a 21-day cycle for up to 6 cycles. Patients could undergo ASCT any time after cycle 2. Following ASCT or completion of combination therapy if not proceeding to ASCT, patients could receive BV monotherapy for up to 16 cycles of total therapy. After a median of 2 cycles of combination therapy (range, 1-6), the objective response rate among 53 efficacy-evaluable patients was 92.5%, with 39 patients (73.6%) achieving CR. Forty patients underwent ASCT. Thirty-one patients (25 of whom underwent ASCT) received BV monotherapy (median, 10 cycles; range, 1-14). After a median of 20.9 months of follow-up, the estimated 2-year progression-free survival was 69.8% and 62.6% for patients who received ASCT and all patients, respectively. Thirty-one patients (56.4%) experienced infusion-related reactions (IRRs), with a majority occurring during cycle 2 of combination therapy. A protocol amendment requiring premedication reduced IRR severity. BV plus bendamustine as first salvage therapy in relapsed/refractory HL is highly active with a manageable toxicity profile. This trial was registered at www.clinicaltrials.gov as #NCT01874054.
Introduction
Although the majority of patients with classical Hodgkin lymphoma (HL) are cured with upfront chemotherapy, up to ∼30% of patients with advanced disease will experience recurrence.1 Patients with disease that is sensitive to salvage chemotherapy typically proceed to high-dose chemotherapy and autologous stem cell transplantation (ASCT) with curative intent.2 Multiple studies have demonstrated that achievement of complete remission (CR) with salvage therapy is one of the strongest predictors of favorable outcome after ASCT.3-7
A number of salvage chemotherapy regimens yield CR rates ranging from ∼20% to 60%.3,4,6-8 The most commonly used regimen in the United States is ifosfamide, carboplatin, and etoposide (ICE) which is typically administered in the hospital setting and is associated with significant myelosuppression, risk of infection, and gastrointestinal toxicity.3,4
Brentuximab vedotin (BV) is an antibody-drug conjugate comprised of an anti-CD30 monoclonal antibody conjugated to the microtubule-disrupting agent monomethyl auristatin E. In heavily pretreated patients whose disease recurred following ASCT, the overall and CR rates associated with single-agent BV treatment were 72% and 33% respectively.9-11 The median progression free survival (PFS) was 9.3 months with a median duration of remission (DOR) of at least 20.5 months for patients achieving a CR. In the second-line setting prior to ASCT, single-agent BV had similar activity with CR rates of 27% to 35%.12,13 Notable toxicities associated with BV included peripheral sensory neuropathy that usually resolved or improved and neutropenia. Bendamustine, an alkylating agent with clinical activity and acceptable tolerability in relapsed non-HL, was also evaluated in relapsed/refractory HL in the post-ASCT setting.14 The overall response and CR rates were 53% and 33%, respectively, with PFS and DOR of ∼5 months each. Significant toxicities associated with bendamustine for the treatment of HL included thrombocytopenia, anemia, and infection.
Given the efficacy and nonoverlapping toxicity profiles of BV and bendamustine, both of which are administered in the outpatient setting, we aimed to evaluate the safety and activity of this combination in patients with primary refractory or first relapse of HL.
Patients and methods
Study design and population
This was a phase 1/2, single-arm, open-label study. Eligible patients were at least 18 years of age with a histopathological diagnosis of classical HL (excluding nodular lymphocyte-predominant HL) and bidimensional measurable disease of at least 1.5 cm along the longest axis at baseline. Patients must have had relapsed or refractory disease following standard frontline chemotherapy. Additional eligibility criteria included: an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 and adequate organ function. Patients with prior exposure to BV or bendamustine, as well as those who had received prior salvage therapy (including salvage radiotherapy), were excluded from the study.
This was a multicenter study conducted at 13 sites across North America. In accordance with the Declaration of Helsinki, the study was approved by the institutional review board of each participating center, and written informed consent was obtained from all patients prior to enrollment.
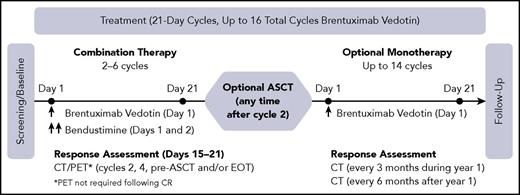
The study consisted of a combination therapy period (a minimum of 2 cycles was required for patients to be efficacy-evaluable), followed by optional ASCT and/or BV monotherapy (a maximum of 16 total cycles BV were permitted). During the combination therapy period, patients received outpatient IV infusions of BV on day 1 and bendamustine (TREANDA was the only formulation used in this study) on days 1 and 2 of a 3-week cycle for up to 6 cycles (Figure 1). Bendamustine was administered after BV on days when both were given.
Study design. CT, computed tomography; EOT, End-of-Treatment; PET, positron emission tomography.
Study design. CT, computed tomography; EOT, End-of-Treatment; PET, positron emission tomography.
White blood cell growth factor and antiemetic usage per institutional guideline were allowable and dose modifications for adverse events (AEs) were recommended. At the discretion of the treating investigator, patients could go off study to undergo ASCT at any time after cycle 2. Hematopoietic stem cell mobilization and collection was performed according to institutional standards. At the discretion of the investigator, patients who continued to meet enrollment criteria could re-enter the study after ASCT to continue treatment with BV as monotherapy. In addition, patients who did not undergo ASCT could remain on study following completion of combination therapy to continue treatment with BV monotherapy. A maximum of 16 total cycles of BV were permitted over the course of the study (combination and monotherapy).
Phase 1 was designed to determine the recommended dose of bendamustine in combination with BV 1.8 mg/kg, and to assess the safety and tolerability of the combination. At least 10 patients were planned for enrollment in this phase. Patients received 1.8 mg/kg BV in combination with a starting dose of 90 mg/m2 bendamustine (Figure 1). The bendamustine dose was to be de-escalated if at least 4 patients experienced a dose-limiting toxicity, defined as any cycle 1 toxicity requiring a dose delay of at least 14 days. Phase 1 was also designed to ensure that there was an acceptable level of activity of the combination regimen and required that at least 2 of the first 10 efficacy evaluable patients achieve a CR as best response in order to progress to phase 2 of the study.
Phase 2 was designed to assess the activity of BV in combination with bendamustine at the recommended tolerable dose determined in phase 1. Approximately 40 additional patients were planned for enrollment into phase 2. A total of 50 efficacy evaluable patients provided ∼90% power for excluding a null hypothesis of a CR rate ≤ 30% with control of type I error at 5%, in the case of the true CR rate being ≥ 50%.
Study assessments
Response was assessed by Investigators using the 2007 Revised Response Criteria for Malignant Lymphoma.15 During combination therapy, assessments used computed tomography (CT) and positron emission tomography (PET) until CR was achieved, after which PET scans were no longer required. Scans were also required at the pre-ASCT and End-of-Treatment visits if none had been performed in the prior 6 weeks. Following completion of combination therapy, CT scans were performed every 3 months for the first year, and every 6 months for the duration of follow-up.
Safety assessments were performed at baseline and on day 1 of each treatment cycle. AEs were monitored from baseline through the End-of-Treatment visit. Anti-therapeutic antibody (ATA) status was assessed at baseline, cycle 2, prior to ASCT (for patients who initiated ASCT), and at the end of treatment.
Statistical analysis
The primary efficacy endpoint was the CR rate during combination therapy. The efficacy-evaluable population included those patients who received at least 2 cycles of combination treatment and had at least 1 post-baseline response assessment before any new antitumor treatment, as well as patients with disease progression after the first dose of combination therapy and before any new antitumor treatment.
Secondary efficacy endpoints included overall best response rate, DOR, and PFS. Additional prespecified exploratory endpoints included overall survival and the feasibility of stem cell mobilization after treatment with BV and bendamustine. Best response rate calculations included all assessments during combination therapy up to the start of another antitumor treatment, including ASCT, and were summarized with exact binomial 95% confidence intervals (CIs). Time to event variables were summarized using Kaplan-Meier estimates with 95% CIs determined using a complementary log-log transformation method. PFS was defined as the time from the first dose of combination therapy to disease progression/relapse or to death from any cause. DOR was defined as the time from first observation of remission to disease progression/relapse or death from any cause. Patients with no subsequent disease progression/relapse or death on study were censored at the time of the last on-study disease assessment demonstrating a lack of progression/relapse. Patients who started a new antitumor treatment (excluding ASCT) were censored at the date of last disease assessment prior to initiation of the new treatment.
Safety assessments were conducted using data from all patients who were enrolled and received at least 1 dose of BV. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.1 and event severity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Results
Patient characteristics and treatment
A total of 55 patients with classical HL (49 nodular sclerosing, 4 mixed cellularity, 1 lymphocyte rich, and 1 with missing subtype) were enrolled between June 2013 and October 2014. Fifty patients (90.9%) had received ABVD as their frontline therapy. Forty-five patients (82%) were at high risk for relapse following ASCT based on the presence of 1 or more of the following features: primary refractory disease, initial remission < 1 year, and/or extranodal disease at time of enrollment. Fifteen patients (27.3%) had stage IV disease at initial diagnosis. Additional baseline demographic data and clinical features are summarized in Table 1.
Patient characteristics
| Characteristic . | N = 55 . |
|---|---|
| Age, median (range), y | 36 (19-79) |
| Male, n (%) | 24 (43.6) |
| White, n (%) | 46 (83.6) |
| ECOG status, n (%) | |
| 0 | 36 (65.5) |
| 1 | 18 (32.7) |
| 2 | 1 (1.8) |
| Months since HL diagnosis, median (range) | 13.8 (3-98) |
| Disease stage at diagnosis, n (%) | |
| I | 3 (5.5) |
| II | 23 (41.8) |
| III | 14 (25.5) |
| IV | 15 (27.3) |
| Frontline therapy received, n (%)* | |
| ABVD† | 50 (90.9) |
| Stanford V | 3 (5.5) |
| AVD | 1 (1.8) |
| VAMP | 1 (1.8) |
| Response to frontline therapy, n (%) | |
| Primary refractory‡ | 28 (50.9) |
| Relapsed | 27 (49.1) |
| CR > 1 y | 17 |
| CR ≤ 1 y | 10 |
| Prior cancer-related radiotherapy, n (%) | 15 (27.3) |
| Baseline disease characteristics, n (%) | |
| B symptoms | 12 (21.8) |
| Bulky disease§ | 5 (9.1) |
| Extranodal disease | 17 (30.9) |
| Bone marrow involvement | 9 (16.4) |
| Characteristic . | N = 55 . |
|---|---|
| Age, median (range), y | 36 (19-79) |
| Male, n (%) | 24 (43.6) |
| White, n (%) | 46 (83.6) |
| ECOG status, n (%) | |
| 0 | 36 (65.5) |
| 1 | 18 (32.7) |
| 2 | 1 (1.8) |
| Months since HL diagnosis, median (range) | 13.8 (3-98) |
| Disease stage at diagnosis, n (%) | |
| I | 3 (5.5) |
| II | 23 (41.8) |
| III | 14 (25.5) |
| IV | 15 (27.3) |
| Frontline therapy received, n (%)* | |
| ABVD† | 50 (90.9) |
| Stanford V | 3 (5.5) |
| AVD | 1 (1.8) |
| VAMP | 1 (1.8) |
| Response to frontline therapy, n (%) | |
| Primary refractory‡ | 28 (50.9) |
| Relapsed | 27 (49.1) |
| CR > 1 y | 17 |
| CR ≤ 1 y | 10 |
| Prior cancer-related radiotherapy, n (%) | 15 (27.3) |
| Baseline disease characteristics, n (%) | |
| B symptoms | 12 (21.8) |
| Bulky disease§ | 5 (9.1) |
| Extranodal disease | 17 (30.9) |
| Bone marrow involvement | 9 (16.4) |
ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; AVD, adriamycin, vinblastine, dacarbacine; BEACOPP, bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone; CHOP, cyclophosphamide, adriamycin, vincristine, predinsone; GVD, gemcitabine, vinorelbine, doxorubicin; PD, progressive disease; PR, partial remission; SD, stable disease; VAMP, vinblastine, adriamycin, methotrexate, prednisone.
Number of cycles of frontline therapy was not determined.
One patient received GVD following ABVD, 1 patient received BEACOPP following ABVD, and 1 patient received ABVD following CHOP.
Response to frontline therapy among patients with primary refractory disease included PR (15 patients), PD (10 patients), SD (2 patients), and CR (1 patient).
As assessed per investigator.
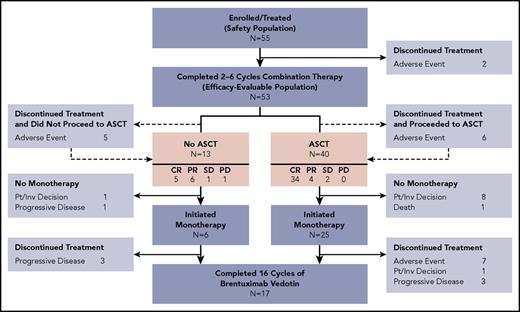
No dose-limiting toxicities were observed during the phase 1 portion of the study, and 90 mg/m2 of bendamustine was determined to be the phase 2 dose. Patient disposition is illustrated in Figure 2. All 55 patients received at least 1 cycle of combination therapy with a median of 2 cycles (range, 1-6). Median dose intensity during combination therapy was 100% (range, 70.6%-102.4%) for BV and 100% (range, 66.7%-102.4%) for bendamustine. A total of 14 patients (25.5%) received at least 1 BV and/or bendamustine dose modification due to AEs during combination therapy (7 patients had dose delays, 3 patients had dose reductions, and 6 patients had an infusion interrupted or stopped early).
Patient disposition. Boxes highlighted in pink indicate best response to combination therapy among subpopulations of patients who did and did not proceed to ASCT. Efficacy-evaluable patients who discontinued treatment owing to AEs during combination therapy are indicated by dashed lines; patients who completed combination therapy but did not enroll in the brentuximab monotherapy phase are shown separately.
Patient disposition. Boxes highlighted in pink indicate best response to combination therapy among subpopulations of patients who did and did not proceed to ASCT. Efficacy-evaluable patients who discontinued treatment owing to AEs during combination therapy are indicated by dashed lines; patients who completed combination therapy but did not enroll in the brentuximab monotherapy phase are shown separately.
Two patients discontinued therapy due to AEs after 1 cycle of combination therapy and were not efficacy-evaluable. Of the 53 efficacy-evaluable patients, 40 underwent ASCT after a median of 2 (range, 2-6) cycles of combination therapy. Thirty-one patients, 25 of whom underwent ASCT, received additional therapy with single-agent BV for a median of 10 cycles (range, 1-14). Median dose intensity during BV monotherapy was 93% (range, 33.1%-100.7%), with 14 patients (45.2%) receiving at least 1 dose modification due to AEs during BV monotherapy (9 patients had dose delays, 8 patients had dose reductions, and 1 patient had an infustion stopped early). Seventeen patients completed 16 total cycles of BV. Six patients underwent consolidative radiotherapy following ASCT, including 4 who also received BV monotherapy.
Treatment response
Response to combination therapy is summarized in Table 2. Of the 53 efficacy-evaluable patients, 39 (73.6%) achieved a best response of CR. The objective response rate (CR plus partial remission [PR]) was 92.5% overall, including 85.7% among patients with primary refractory HL and 100% among patients with relapsed disease. The objective responses rates were 95.0% and 84.6% in patients who did and did not undergo transplantation, respectively. Among 14 efficacy-evaluable patients with stage IV disease at diagnosis, the CR and objective response rates were 64.3% and 85.7%, respectively.
Best response on combination therapy
| Population . | Best clinical response, n (%) [95% CI] . | ||||
|---|---|---|---|---|---|
| CR . | PR . | SD . | PD . | ORR* . | |
| Overall, N = 53 | 39 (73.6) [59.7, 84.7] | 10 (18.9) | 3 (5.7) | 1 (1.9) | 49 (92.5) [81.8, 97.9] |
| Response to frontline therapy | |||||
| Primary refractory, n = 28 | 18 (64.3) [44.1, 81.4] | 6 (21.4) | 3 (10.7) | 1 (3.6) | 24 (85.7) [67.3, 96.0] |
| Relapsed, n = 25 | 21 (84.0) [63.9, 95.5] | 4 (16.0) | 0 (0.0) | 0 (0.0) | 25 (100) [86.3, 100] |
| ASCT | |||||
| Yes, n = 40 | 34 (85.0) [70.2, 94.3] | 4 (10.0) | 2 (5.0) | 0 (0.0) | 38 (95.0) [83.1, 99.4] |
| No, n = 13 | 5 (38.5) [13.9, 68.4] | 6 (46.2) | 1 (7.7) | 1 (7.7) | 11 (84.6) [54.6, 98.1] |
| Population . | Best clinical response, n (%) [95% CI] . | ||||
|---|---|---|---|---|---|
| CR . | PR . | SD . | PD . | ORR* . | |
| Overall, N = 53 | 39 (73.6) [59.7, 84.7] | 10 (18.9) | 3 (5.7) | 1 (1.9) | 49 (92.5) [81.8, 97.9] |
| Response to frontline therapy | |||||
| Primary refractory, n = 28 | 18 (64.3) [44.1, 81.4] | 6 (21.4) | 3 (10.7) | 1 (3.6) | 24 (85.7) [67.3, 96.0] |
| Relapsed, n = 25 | 21 (84.0) [63.9, 95.5] | 4 (16.0) | 0 (0.0) | 0 (0.0) | 25 (100) [86.3, 100] |
| ASCT | |||||
| Yes, n = 40 | 34 (85.0) [70.2, 94.3] | 4 (10.0) | 2 (5.0) | 0 (0.0) | 38 (95.0) [83.1, 99.4] |
| No, n = 13 | 5 (38.5) [13.9, 68.4] | 6 (46.2) | 1 (7.7) | 1 (7.7) | 11 (84.6) [54.6, 98.1] |
ORR, objective response rate. Other abbreviations are explained in Table 1.
CR plus PR.
Thirty-four of the 39 patients (87.2%) with a best response of CR achieved this response at cycle 2, including 30 of 34 patients (88.2%) who underwent ASCT with a CR. Twenty-one of these 30 patients (70%) proceeded to transplant without additional cycles of combination therapy, while 9 patients (30%) received from 1 to 4 additional cycles prior to transplant. Five patients achieved CR but did not proceed to ASCT; 1 lacked insurance coverage for transplant, 1 was not an ASCT candidate due to age and/or comorbidities, 1 switched to an alternate salvage therapy due to AE, and 2 were patient decisions. Of 6 patients who underwent ASCT with a best response of PR or stable disease (SD), 4 achieved a CR subsequent to transplant.
Stem cell mobilization and transplant
Forty-one patients initiated hematopoietic stem cell mobilization; of these, 39 (95.1%) underwent successful peripheral blood collection with the first attempt (supplemental Table 1, available on the Blood Web site). Thirty-seven of the 41 patients (90.2%) received initial stimulation with granulocyte colony-stimulating factor (G-CSF) with or without plerixafor and 4 of the 41 patients (9.8%) received cyclophosphamide with G-CSF. One patient with inadequate mobilization following initial stimulation with G-CSF plus plerixafor underwent successful stem cell harvest after additional plerixafor. The second patient who failed to mobilize stem cells after initial stimulation with G-CSF alone underwent a successful bone marrow harvest. One of the 41 patients experienced progressive disease after stem cell collection and received additional salvage treatment instead of ASCT. Of the 40 patients who proceeded to ASCT, 30 (75.0%) received BEAM (carmustine, etoposide, cytarabine, and melphalan) as their conditioning regimen. Thirty-nine of the 40 patients who underwent ASCT successfully engrafted (median times to neutrophil and platelet engraftment were 12 and 14 days, respectively); however, 1 patient died of septic shock prior to engraftment.
Long-term follow-up
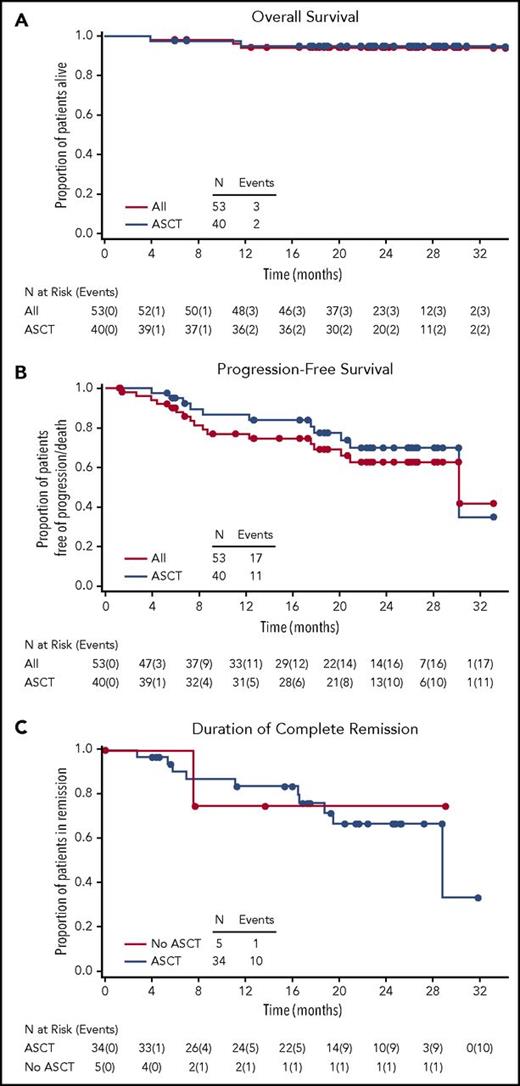
With a median follow-up time of 23.0 (range, 4-34) months from initiation of combination therapy and 21.3 (range, 0-30) months from ASCT, the estimated 2-year overall survival (OS) was 94.9% (95% CI, 81.0%-98.7%) for those patients who underwent transplantation and 94.2% (95% CI, 83.0%-98.1%) overall (Figure 3A). In addition to the patient who died prior to stem cell engraftment, 2 patients who did not undergo ASCT succumbed to progressive disease during follow-up. None of the deaths were considered treatment-related, as all occurred in patients who had been off study treatment of at least 30 days.
Kaplan-Meier analyses. (A) Overall survival for all patients and for those who underwent ASCT. (B) Progression-free survival for all patients and for those who underwent ASCT. (C) Duration of complete remission according to ASCT status.
Kaplan-Meier analyses. (A) Overall survival for all patients and for those who underwent ASCT. (B) Progression-free survival for all patients and for those who underwent ASCT. (C) Duration of complete remission according to ASCT status.
Median follow-up times for PFS were 20.9 (range, 1-33) months from initiation of combination therapy and 19.2 (range, 0-30) months from ASCT. The 2-year PFS was 69.8% (95% CI, 50.6%-82.7%) for those who underwent ASCT and 62.6% (95% CI, 45.7%-75.6%) overall (Figure 3B). Duration of CR was similar among patients who did and did not undergo ASCT (Figure 3C).
Adverse events
Treatment-emergent AEs are summarized in Table 3 and supplemental Table 2. A total of 31 patients (56.4%) experienced a grade 3 or 4 toxicity. These included lymphopenia (n = 6 [10.9%]), rash (maculopapular rash, n = 5 [9.1%], generalized rash, n = 2 [3.6%], erythematous rash, n = 1 [1.8%], pruritic rash, n = 1 [1.8%]), and hypotension (n = 4 [7.3%]). Overall, 20 patients (36.4%) discontinued therapy due to AEs, 18 of whom were efficacy-evaluable. Of these, 14 (77.8%) had achieved a CR (all by cycle 2) and 13 (72.2%; 12 CR and 1 SD) proceeded to ASCT.
Treatment-emergent adverse events
| Patients with event . | Combination therapy, N = 55 . | BV monotherapy, N = 31 . |
|---|---|---|
| n (%) . | ||
| Any TEAE | 54 (98.2) | 28 (90.3) |
| Grade 3/4 TEAEs | 26 (47.3) | 8 (25.8) |
| SAEs | 13 (23.6) | 3 (9.7) |
| TEAEs leading to treatment discontinuation | 13 (23.6) | 8 (25.8) |
| TEAEs leading to dose modification | 14 (25.5) | 14 (45.2) |
| Dose delay | 7 (12.7) | 9 (29.0) |
| Dose reduction | 3 (5.5) | 8 (25.8) |
| Infusion interruption or early termination | 6 (10.9) | 1 (3.2) |
| Infusion-related reactions | 31 (56.4) | 7 (12.7) |
| Peripheral neuropathy | 13 (23.6) | 21 (67.7) |
| Febrile neutropenia* | 0 (0.0) | 1 (3.2) |
| Patients with event . | Combination therapy, N = 55 . | BV monotherapy, N = 31 . |
|---|---|---|
| n (%) . | ||
| Any TEAE | 54 (98.2) | 28 (90.3) |
| Grade 3/4 TEAEs | 26 (47.3) | 8 (25.8) |
| SAEs | 13 (23.6) | 3 (9.7) |
| TEAEs leading to treatment discontinuation | 13 (23.6) | 8 (25.8) |
| TEAEs leading to dose modification | 14 (25.5) | 14 (45.2) |
| Dose delay | 7 (12.7) | 9 (29.0) |
| Dose reduction | 3 (5.5) | 8 (25.8) |
| Infusion interruption or early termination | 6 (10.9) | 1 (3.2) |
| Infusion-related reactions | 31 (56.4) | 7 (12.7) |
| Peripheral neuropathy | 13 (23.6) | 21 (67.7) |
| Febrile neutropenia* | 0 (0.0) | 1 (3.2) |
SAE, serious adverse event; TEAE, treatment-emergent adverse event.
A total of 18 patients received prophylactic growth factor support during the study, 9 of whom received primary prophylaxis.
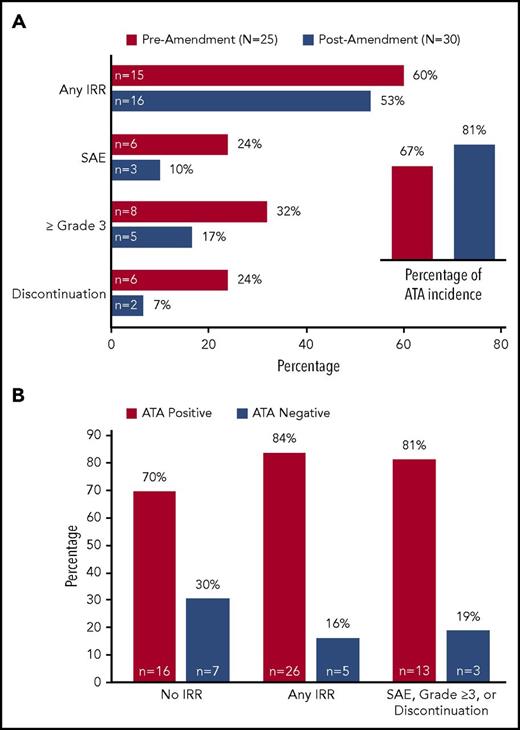
Notably, many of the adverse effects in the study were attributed to infusion related reactions (IRRs), which occurred in 31 patients (56.4%), and were considered serious in 9 patients (14.5%). The majority of IRRs occurred during cycle 2 of combination therapy (28 of 31 patients), although 9 patients experienced IRRs during cycle 1. In addition, IRRs were reported in 4 patients during cycles 3-6 of combination therapy and in 7 patients during monotherapy. The most common IRR symptoms (of any grade) were fever (25.5%), chills (20.0%), dyspnea (16.4%), flushing (14.5%), nausea (14.5%), hypotension (10.9%), and pruritus (10.9%). Importantly, there were no cases of anaphylaxis. The incidence of IRRs in 15 of the first 25 patients treated (60.0%) exceeded rates of ∼12% to 15% reported for the individual drugs.16,17 Of these 25 patients, 23 had received prophylactic glucocorticoids for a variety of indications, including management of nausea and IRRs. Therefore, the study was amended to require high-dose corticosteroid and antihistamine premedication (100 mg methylpredinsone or equivalent and 50 mg diphenhydramine or equivalent were recommended) with combination therapy. This approach decreased the severity of the IRRs but did not appreciably change the incidence (Figure 4A). Delayed hypersensitivity reactions with onset >24 hours after infusion occurred in 24 patients (43.6%), and were grade 3 in 7 patients (12.7%). Delayed hypersensitivity reactions were characterized by fever, rash (maculopapular and generalized), chills, nausea and pruritus.
Influence of premedication and ATA status on infusion-related reactions. (A) Incidence of IRRs by category (all IRRs and those reported as SAEs, grade 3/4, or leading to treatment discontinuation) among patients in the safety population (N = 55), according to whether the first dose of combination therapy was administered before or after a protocol amendment requiring premedication with antihistamines and corticosteroids. Inset, ATA incidence among immunogenicity-evaluable patients (N = 48) before and after the amendment. (B) ATA status of patients in each IRR category in the population of 54 patients with any ATA assessment (42 ATA+ at any time during the study and12 ATA− throughout the study). IRR, infusion-related reaction; SAE, serious adverse event.
Influence of premedication and ATA status on infusion-related reactions. (A) Incidence of IRRs by category (all IRRs and those reported as SAEs, grade 3/4, or leading to treatment discontinuation) among patients in the safety population (N = 55), according to whether the first dose of combination therapy was administered before or after a protocol amendment requiring premedication with antihistamines and corticosteroids. Inset, ATA incidence among immunogenicity-evaluable patients (N = 48) before and after the amendment. (B) ATA status of patients in each IRR category in the population of 54 patients with any ATA assessment (42 ATA+ at any time during the study and12 ATA− throughout the study). IRR, infusion-related reaction; SAE, serious adverse event.
Reversible peripheral neuropathy is a known and common effect of BV treatment. In the current study, peripheral neuropathy was reported in 30 patients (54.4%) overall, with 13 of 55 patients (23.6%) experiencing symptoms during combination therapy and 21 of 31 patients (67.7%) experiencing symptoms during monotherapy. Peripheral neuropathy led to treatment discontinuation in 4 (7.3%) patients (1 during combination therapy and 3 during monotherapy) and was the most common reason for dose reduction and delay during the monotherapy portion of the study. Nearly all cases of peripheral neuropathy were grade 1-2, except for 1 patient who experienced grade 3 muscular weakness during combination therapy and 1 patient with grade 3 motor neuropathy during monotherapy. There was a single episode of grade 3 febrile neutropenia during BV monotherapy; no additional severe neutropenic events were reported. Eighteen of the 55 patients who received combination therapy (32.7%) received prophylactic growth factor support at least once during treatment, with 9 patients (16.4%) receiving primary prophylaxis (defined as growth factor support given by day 5 of treatment initiation).
Immunogenicity
Fifty-one patients had their BV antitheraputic antibody (ATA) status assessed at both baseline and at least 1 postbaseline visit. Of these, 48 were ATA-negative and 3 were ATA-positive at baseline. Among the 48 baseline ATA-negative patients, the post-treatment ATA incidence was 75.0% (36 of 48 patients) overall, and was greater at the cycle 2 visit compared with the end of treatment visit (71.1% vs 30.6%). Twelve of the 48 baseline ATA-negative patients remained ATA-negative at all post-baseline assessments. The CR rates among 46 of the 48 baseline ATA-negative patients who were efficacy-evaluable were 70.6% (24 of 34 patients) for ATA-incident patients and 83.3% (10 of 12 patients) for persistently ATA-negative patients. Three additional patients had no ATA assessment at baseline but had an ATA-positive assessment at at least 1 post-baseline visit. The relationship between ATA status and IRRs among all 54 patients with an ATA assessment at any time during the study is illustrated in Figure 4B.
Discussion
Overall, the combination of BV and bendamustine as first salvage therapy for patients with relapsed/refractory HL was associated with high CR rates (72.6%) and overall response rates (92.5%). Moreover, 87% of patients who achieved CR did so after only 2 cycles of therapy. In general, the combination was well tolerated. Although the rate of IRRs was significant, the severity of the reactions was mitigated by premedication with high-dose corticosteroids and antihistamines and the vast majority of patients were able to receive at least 2 cycles of therapy. Increased immunogenicity of BV was apparent with the combination relative to historical experience with monotherapy (ATA incidence 75% and ∼37%, respectively).16 Although the basis for increased immunogenicity is unclear, the coincident timing of maximum ATA-positivity and IRRs at cycle 2 suggests an immune-mediated mechanism for IRRs. Notably, outcomes were not markedly affected by either ATA status or IRRs. The incidence of peripheral neuropathy was generally in line with expectations, particularly in the setting of prior exposure to vinblastine, and was manageable for the majority of patients. No patients experienced febrile neutropenia on combination therapy, despite relatively low utilization of G-CSF primary prophylaxis. A single episode of febrile neutropenia was reported during BV monotherapy. Stem cell collection was predictably successful and the PFS and OS rates after ASCT were excellent.
The DOR results in patients achieving a CR to combination therapy appeared similar for the small number of patients who did not go on to ASCT and the majority of patients who did. Although the impact of BV consolidation after salvage is difficult to assess in this setting given the nonrandomized study design and the small number of patients who did not receive maintenance therapy, the AE profile during monotherapy was generally consistent with results from a phase 3 trial of BV in the post-ASCT setting, in which patients received similar duration of monotherapy (median, 9 cycles).18
Our results were particularly favorable with respect to CR rate, which is a strong, and perhaps the most robust, predictor of PFS post-ASCT. It is difficult to directly compare regimens in the absence of a randomized controlled trial. However, standard salvage chemotherapy regimens such as ICE, cytosine arabinoside, cisplatin, and dexamethasone (DHAP), and gemcitabine, vinorelbine, and liposomal doxorubicin (GVD) have been associated with high overall response rates and CR rates ranging from ∼20% to 60%.3,4,7 The variability of response rates across studies may, in part, be explained by the use of different response assessment criteria over time. Our study used the 2007 Revised Response Criteria for Malignant Lymphoma which requires that fluorodeoxyglucose uptake at sites of residual masses be lower than or equal to that of the mediastinal blood pool for a PET scan to be considered negative.19 In addition to the high CR rate, the combination of BV and bendamustine is an attractive salvage regimen because it does not require inpatient administration and was associated with low rates of myelosuppression and infection. Results from other studies have also demonstrated activity of the combination in HL, both in heavily pretreated patients and in patients with primary refractory disease.20,21
Two prior studies evaluated the role of single-agent BV as salvage therapy in patients with relapsed/refractory HL after frontline treatment. Chen et al treated 37 patients with single-agent BV for 4 doses given every 3 weeks.12 The overall response and CR rates were 68% and 35%, respectively. Moskowitz and colleagues administered BV monotherapy in 45 patients weekly for 8 doses followed by PET restaging.13 Patients who achieved CR proceeded to transplant and those with less than CR received 2 cycles of augmented ICE. After BV monotherapy, 27% of patients were PET negative and 76% of patients overall achieved a negative PET. Although arguably a stepwise approach is appealing in order to avoid chemotherapy, the CR rates were low with BV monotherapy and therefore this strategy resulted in a delay in getting many patients to ASCT.
Recently, other combinations with bendamustine have also demonstrated excellent outcomes. Santoro and colleagues published results of a phase 2 study of 59 relapsed/refractory HL patients with characteristics similar to our patient population.22 Patients were treated with BeGV; gemcitabine (800 mg/m2 on days 1 and 4), vinorelbine (20 mg/m2 on day 1), bendamustine (90 mg/m2 days 2 and 3) and prednisolone (100 mg on days 1-4) for 4 cycles. The overall and CR rates were 83% and 73%, respectively. With a median follow-up 29.1 months, the 2-year PFS was 62.2%, comparable to results reported here. The 2-year OS for BeGV was 77.6%. Notably, in the current study, 2 cycles of BV and bendamustine were sufficient for most patients to achieve a CR. In a recent study of previously untreated patients aged 60 and over who were not candidates for standard anthracycline based chemotherapy, the combination of brentuximab and bendamustine was associated with ORR and CR rates of 100% and 88%. The combination, however, was felt to be too toxic in this patient population and this arm of the trial closed early.23
Limitations of our trial include the single-arm study design, which makes efficacy comparison with other salvage regimens difficult. Furthermore, follow-up is currently relatively brief, so additional time will be required to fully assess long-term PFS. In addition, this study was not designed to assess the impact of BV monotherapy post-ASCT in patients treated with BV and bendamustine prior to ASCT.
In conclusion, the combination of BV and bendamustine as first-line salvage therapy in relapsed/refractory HL is highly active and has a manageable toxicity profile, with the vast majority of patients proceeding to ASCT after just 2 cycles of therapy. Future studies could build on this trial by adding other active agents to the regimen to further enhance activity and by evaluating whether subsets of patients might achieve long-term disease control without the need for high dose chemotherapy and ASCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and clinical study teams who participated in this research. Medical writing and editorial support were provided by Eric Bertelsen, an employee of Seattle Genetics, Inc.
This work was supported by research funding from Seattle Genetics, Inc.
Authorship
Contribution: A.S.L. and R.A. contributed to the conception and design of the study, data collection and interpretation, and writing of the report; J.V. and O.A.O. contributed to the conception and design of the study, data interpretation, and critical review of the report; R.G.B., A.S., P.C., E.A., J.M., S.M.A., H.E.C., M.I.-O., C.B., E.C., and A.F.-T. contributed to the data and interpretation and critical review of the report; N.J. contributed to the study design, data interpretation, and writing of the report; Y.W. contributed to the study design, data interpretation, and critical review of the report.
Conflict-of-interest disclosure: The institutions of A.S.L., R.G.B., A.S., P.C., E.A., J.M., S.M.A., H.E.C., M.I.-O., C.B., E.C., A.F.-T., J.V., O.A.O., and R.A. received funding from Seattle Genetics, Inc. to conduct the trial. H.E.C. and N.J. have equity ownership in Seattle Genetics, Inc. J.V. and A.S. have received honoraria from Seattle Genetics, Inc. J.M. and C.B. have participated in a speakers’ bureau for Seattle Genetics, Inc and Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Limited. O.A.O. has received research funding from Seattle Genetics, Inc. H.E.C. has acted as a consultant for and has received travel expenses from Seattle Genetics, Inc. N.J. and Y.W. are employed by Seattle Genetics, Inc.
Correspondence: Ann S. LaCasce, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: ann_lacasce@dfci.harvard.edu.