Abstract
Hemophilia and von Willebrand disease are the most common congenital bleeding disorders. Treatment of these disorders has focused on replacement of the missing coagulation factor to prevent or treat bleeding. New technologies and insights into hemostasis have driven the development of many promising new therapies for hemophilia and von Willebrand disease. Emerging bypass agents including zymogen-like factor IXa and Xa molecules are in development and a bispecific antibody, emicizumab, demonstrated efficacy in a phase 3 trial in people with hemophilia A and inhibitors. Tissue factor pathway inhibitor, the protein C/S system, and antithrombin are targets of novel compounds in development to alter the hemostatic balance and new approaches using modified factor VIII molecules are being tested for prevention and eradication of inhibitor antibodies in hemophilia A. The first recombinant von Willebrand factor (VWF) product has been approved and has unique VWF multimer content and does not contain factor VIII. These new approaches may offer better routes of administration, improved dosing regimens, and better efficacy for prevention and treatment of bleeding in congenital bleeding disorders.
Introduction
Hemophilia A (factor VIII [FVIII] deficiency) and B (FIX deficiency) are the most common severe bleeding disorders, affecting 1 in 5000 and 1 in 20 000 males, respectively.1 Von Willebrand disease (VWD) is the most common congenital bleeding disorder, affecting 1 in 1000 people and up to 1 in 100 with low von Willebrand factor (VWF) levels. The treatment of bleeding disorders has advanced markedly over the past 2 centuries. In 1840, whole blood transfusion was pioneered by Lane and became a rarely used and only minimally effective treatment of bleeding disorders.2 The concept of replacing what was missing in the blood was prescient, but the quantities of specific clotting factors delivered in whole blood were insufficient. In the 1950s and 1960s, the advent of plasma fractionation and Judith Graham Pool’s discovery that FVIII and VWF were present in high concentrations in the precipitate of frozen plasma led to major improvements in replacement therapy for hemophilia and VWD.3 In the 1970s, the discovery that desmopressin released stored pools of VWF and FVIII led to a novel treatment of mild hemophilia and VWD.4 Plasma fractionation innovations allowed the development of highly enriched FVIII, FIX, and VWF concentrates, ushering in a golden era of treatment and an important shift from episodic or “on demand” replacement therapy in response to bleeding to regular prophylactic replacement therapy, leading to highly effective bleed prevention and improved clinical outcomes. Tragically, the requirement of many plasma donors for production of clotting factor concentrates lead to widespread HIV and viral hepatitis infection in persons with hemophilia in the 1980s. This led to improved viral testing and inactivation methodologies for plasma-derived products, followed by the development of recombinant replacement proteins that virtually eliminated the risk of these infections. Bioengineering innovations have led to the development of recombinant FVIII and FIX therapeutics with extended half-life. These recombinant extended half-life therapies may be used to reduce the burden of prophylaxis by reducing the frequency of prophylactic administration or to maintain higher plasma levels to achieve better hemostatic efficacy. Recent hemophilia gene therapy clinical trials have demonstrated that replacement therapy can be achieved perpetually after liver-directed targeting with adeno-associated viral vectors with potentially curative levels of FVIII and FIX achieved that have been durable to date with good short-term safety profiles.5
The focus for all of these hemophilia treatments over the past 2 decades has been based on replacement of the missing coagulation protein. However, recombinant technology combined with improved basic understanding of coagulation biochemistry is shifting the treatment paradigm. Novel therapeutics have been developed with alternative modes of delivery, novel targets that overcome the limitations of current replacement therapy, and markedly improve pharmacokinetic profiles with a very low burden of administration. These new therapies promise to transform our approaches to not only hemophilia, but also many other bleeding disorders (Figure 1).
Mechanisms of novel hemophilia therapies. (A) Normal hemostatic balance tipped in favor of bleeding, for example, (B) in hemophilia A from lack of coagulation FVIII. (C) One approach to improve hemostatic balance in hemophilia is to add additional procoagulants; (D) another approach is to remove or inhibit anticoagulants. Adapted from Willyard.64
Mechanisms of novel hemophilia therapies. (A) Normal hemostatic balance tipped in favor of bleeding, for example, (B) in hemophilia A from lack of coagulation FVIII. (C) One approach to improve hemostatic balance in hemophilia is to add additional procoagulants; (D) another approach is to remove or inhibit anticoagulants. Adapted from Willyard.64
Emerging bypassing agents
In 1908, Paul Ehrlich described antibodies as the body’s silver bullets to target proteins precisely and specifically. In 1984, Kohler, Jerne, and Milstein were awarded the Nobel Prize for discovery of monoclonal antibodies. In 2012, Kitazawa published the compilation of nearly a decade of development of a bispecific antibody with 1 Fab unit binding the active enzyme, FIXa, and the other binding the zymogen FX, uniting the coagulation factors together in the proper conformation for activation of FX.6 Initially unsuccessful and limited by the potential for clearance of FIX and FX through antibody binding, the Kitazawa group developed 200 anti-FIX and 200 anti-FX Fab units and combined them to create a library of 40 000 combinations; they selected the combination with an ideal low binding affinity and superior activity in FXa production in the setting of clot formation. The candidate antibody was tolerated at high doses in animal models and corrected the hemostatic defect in a hemophilia A cynomolgus monkey model.7 The name ACE910 is an acronym for antibody mimicking coagulation factor eight by connecting factor 9 and factor 10. However, the generic name of the antibody is emicizumab (HemLibra, Hoffmann-La Roche AG, Basel, Switzerland). The major advancement over other hemophilia A therapeutics was in its subcutaneous route for administration, long half-life, and increased efficacy in bleed prevention.8 In May 2016, Shima et al reported the results of a 6-month open label phase 1/2 dose escalation study in people with severe hemophilia A with or without inhibitors.9 At all doses, the drug was safe and effective, reducing the annualized bleed rate (ABR) significantly compared with historical bleeding in these patients; 73% of subjects had no bleeds. In July 2017, Oldenberg et al reported the results of a phase 3 open label study of emicizumab in 109 people with hemophilia A and high titer inhibitors.8 The primary analysis, focused on the bleeding rate between subjects randomized to emicizumab (4 weekly 3 mg/kg loading doses followed by weekly maintenance injections of 1.5 mg/kg per dose) compared with a control group treated with on-demand bypassing agents at the time of bleeding, demonstrated 87% fewer bleeds in the emicizumab group (mean ABR, 2.9 vs 23.3). Remarkably, 63% of the emicizumab prophylaxis group had no bleeding compared with 6% of the control group. Secondary analysis compared prospectively collected bleeding data in subjects on bypass agent prophylaxis before initiation of emicizumab to their own bleeding while on emicizumab and demonstrated a 79% reduction in bleeding on emicizumab (mean ABR, 3.3 vs 15.7). Data from interim analysis of the ongoing trial in pediatric patients with inhibitor antibodies have demonstrated a 99% reduction in treated bleeds with pharmacokinetics similar to that observed in adult patients.10 Limited reports of surgery in patients on emicizumab alone or in conjunction with additional clotting factor have been reported, and an observational surgery study is ongoing.11-13 During the trial, there were 5 thrombotic severe adverse events, including 2 thromboses and 3 thrombotic microangiopathy events. All of the events were in the setting of a breakthrough bleed treated with activated prothrombin complex (aPCC) at doses >100 IU/kg per day for more than 24 hours (5/8 treated with such intense regimen), whereas many breakthrough bleeds were treated with activated FVIIa (0/140 treated with activated FVIIa) alone or <100 IU/kg per day or <24 hours treatment with an aPCC and did not develop thrombotic events (0/70 treated with these less intense regimens). Synergistic thrombin generation has been demonstrated with aPCC and emicizumab in vitro and in vivo.14 The cumulative doses of aPCC may lead to accumulation of nonactivated and activated clotting factor substrates (eg, FIXa, FX), enhancing FXa generation in the presence of emicizumab (Figure 2). A mitigation strategy was put in place during A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Prophylactic Emicizumab Versus no Prophylaxis in Hemophilia A Participants With Inhibitors 1 trial; when the mitigation plan was followed, there were no additional thrombotic or thrombotic microangiopathy events on the trial (Table 1).15 All of these data are from studies of patients with hemophilia A and inhibitors; based on these results, emicizumab was approved by the US Food and Drug Administration for the prevention of bleeding episodes in adults and children with hemophilia A and FVIII inhibitors. Studies are ongoing to assess the efficacy and safety of emicizumab in people with hemophilia A without inhibitor antibodies.
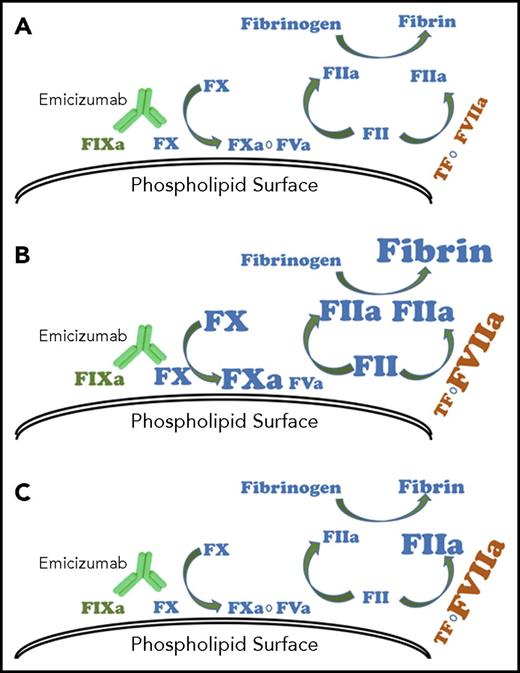
Model for effect of bypassing agents with emicizumab. (A) Emicizumab mimics the function of coagulation FVIIIa in hemostasis. With repeated doses of activated prothrombin complex concentrates, (B) intrinsic, extrinsic, and common pathway procoagulant proteins can accumulate, resulting in exaggerated thrombin generation and fibrin formation. Recombinant FVIIa, because of its short half-life, is unlikely to accumulate and (C) drives increased thrombin generation only through the extrinsic pathway.
Model for effect of bypassing agents with emicizumab. (A) Emicizumab mimics the function of coagulation FVIIIa in hemostasis. With repeated doses of activated prothrombin complex concentrates, (B) intrinsic, extrinsic, and common pathway procoagulant proteins can accumulate, resulting in exaggerated thrombin generation and fibrin formation. Recombinant FVIIa, because of its short half-life, is unlikely to accumulate and (C) drives increased thrombin generation only through the extrinsic pathway.
New therapies for bleeding disorders undergoing investigation in human clinical trials
| Drug . | Target disease(s) . | Mechanism . | Drug class . | Stage of clinical development . | Route of administration pharmacokinetics . | Trial results . |
|---|---|---|---|---|---|---|
| Emicizumab | Hemophilia A with and without inhibitors | FVIIIa mimetic | Bispecific humanized antibody | Phase 3 clinical trials completed | Subcutaneous half-life, 30 d | 62%-71% 0 ABR 79%-87% bleed reduction in inhibitor patients |
| Zymogen-like FXa | Hemophilia A and B with inhibitors, other bleeding disorders, and anticoagulant reversal | Bypass agent | Modified recombinant clotting factor | Phase 1 | Intravenous half-life, 4 min | Improved aPTT, thrombin generation, increased TAT complexes, prothrombin fragment 1 + 2, and D-dimer |
| Zymogen-like FIXa | Hemophilia B | Bypass agent | Modified recombinant clotting factor | Preclinical | Intravenous half-life, h | Improved hemostasis in hemophilia mouse tail clip model |
| FIXa not dependent on FVIIIa cofactor activity | Hemophilia A with inhibitors | Bypass agent | Modified Recombinant clotting factor | Preclinical | Intravenous half-life, h | Shortened aPTT and improved hemostasis in hemophilia mouse tail clip model |
| Fitusiran | Hemophilia A and B with inhibitors and other bleeding disorders | Targeting natural anticoagulant pathway | GalNAC-siRNA | Phase 2 clinical trial ongoing | Subcutaneous, administered monthly | 48% 0 ABR in mixed hemophilia A and B inhibitor population |
| TFPI | Hemophilia A and B with inhibitors and other bleeding disorders | Targeting natural anticoagulant pathway | Monoclonal antibodies, antibody fragment, | Phase 1 clinical trial published | Subcutaneous half-life, 1-5 d | Increased D-dimer and prothrombin fragments |
| Mutant α1 antitrypsin | Hemophilia A and B with inhibitors and other bleeding disorders | Targeting natural anticoagulant pathway | Modified recombinant clotting factor | Preclinical | Intravenous half-life, 5-7 d | Improved thrombin generation and improved hemostasis in hemophilia mouse tail clip model |
| rVWF | Von Willebrand disease | Factor replacement | Recombinant clotting factor | FDA approved | Intravenous half-life, 21.9 h | 96.9% good or excellent bleed control in 192 bleeds |
| Drug . | Target disease(s) . | Mechanism . | Drug class . | Stage of clinical development . | Route of administration pharmacokinetics . | Trial results . |
|---|---|---|---|---|---|---|
| Emicizumab | Hemophilia A with and without inhibitors | FVIIIa mimetic | Bispecific humanized antibody | Phase 3 clinical trials completed | Subcutaneous half-life, 30 d | 62%-71% 0 ABR 79%-87% bleed reduction in inhibitor patients |
| Zymogen-like FXa | Hemophilia A and B with inhibitors, other bleeding disorders, and anticoagulant reversal | Bypass agent | Modified recombinant clotting factor | Phase 1 | Intravenous half-life, 4 min | Improved aPTT, thrombin generation, increased TAT complexes, prothrombin fragment 1 + 2, and D-dimer |
| Zymogen-like FIXa | Hemophilia B | Bypass agent | Modified recombinant clotting factor | Preclinical | Intravenous half-life, h | Improved hemostasis in hemophilia mouse tail clip model |
| FIXa not dependent on FVIIIa cofactor activity | Hemophilia A with inhibitors | Bypass agent | Modified Recombinant clotting factor | Preclinical | Intravenous half-life, h | Shortened aPTT and improved hemostasis in hemophilia mouse tail clip model |
| Fitusiran | Hemophilia A and B with inhibitors and other bleeding disorders | Targeting natural anticoagulant pathway | GalNAC-siRNA | Phase 2 clinical trial ongoing | Subcutaneous, administered monthly | 48% 0 ABR in mixed hemophilia A and B inhibitor population |
| TFPI | Hemophilia A and B with inhibitors and other bleeding disorders | Targeting natural anticoagulant pathway | Monoclonal antibodies, antibody fragment, | Phase 1 clinical trial published | Subcutaneous half-life, 1-5 d | Increased D-dimer and prothrombin fragments |
| Mutant α1 antitrypsin | Hemophilia A and B with inhibitors and other bleeding disorders | Targeting natural anticoagulant pathway | Modified recombinant clotting factor | Preclinical | Intravenous half-life, 5-7 d | Improved thrombin generation and improved hemostasis in hemophilia mouse tail clip model |
| rVWF | Von Willebrand disease | Factor replacement | Recombinant clotting factor | FDA approved | Intravenous half-life, 21.9 h | 96.9% good or excellent bleed control in 192 bleeds |
aPTT, activated partial thromboplastin time; FDA, US Food and Drug Administration; GalNAC, N-acetylgalactosamine; TAT, thrombin antithrombin complexes.
Bypassing the intrinsic pathway in patients with hemophilia and inhibitors has focused on induction of the extrinsic pathway through addition of FVIIa or both the extrinsic and common pathways with aPCC. Although FXa has the potential to bypass the intrinsic pathway and improve thrombus formation, its use has been hampered by the intrinsic short half-life of FXa and its rapid inhibition by endogenous plasma inhibitors.16 Additionally, doses of FXa in excess of the endogenous inhibitors results in unregulated thrombosis. The development of a mutant (I16L) zymogen-like FXa has overcome some of these limitations with improved regulation requiring FVa for factor Xa activity. FVa would be expected to localize to vascular injury only, decreasing interaction with endogenous anticoagulants and improving half-life.17-20 This mutation results in a conformational change that at the same time reduces the active protease conformation in the unbound state, but provides similar activity to wild-type Xa when in the prothrombinase complex.21 These changes lead to improved thrombin generation in hemophilic plasma19 and blood22 and reduce tail clip bleeding mouse models of hemophilia.18 A phase 1 study suggests the half-life of this agent may be only a few minutes, but this could be used as a continuous infusion for significant bleeding.23
A similar approach to modifications of FIX has demonstrated promising properties in vitro and in mouse models of hemophilia B.24 Again, a point mutation at amino acid 16, V16L, resulted in less inhibition by antithrombin, more stable prothrombinase complex, and reduced bleeding in a tail clip murine hemophilia B bleeding model.24 Another approach to modification of FIX has been proposed as a therapy for hemophilia A with inhibitors.25 An FIX variant, V181I/K265T/Y345T, resulted in FIXa activity independent of FVIIIa and was able to achieve 15.6% FVIII activity in vitro by a 1-stage FVIII activity assay. This variant shortened clotting times in hemophilia A inhibitor plasma and when expressed in mouse models of hemophilia A or hemophilia A with inhibitors, improved hemostatic response to tail clip and laser injury.25 In mice tolerant to human FIX, these variants were not immunogenic.
Agents that alter the hemostatic balance
The prevailing paradigm in bleeding disorder management has been to replace the missing procoagulant factor or factors, or when this is not possible, as is the case in hemophilia A with inhibitors, to add other activated procoagulants to bypass the absent factor and promote thrombus formation. More recently, focus has turned to decreasing natural anticoagulants such as tissue factor pathway inhibitor (TFPI), antithrombin, and the protein C/S pathway. The rationale for this approach is that coagulation homeostasis is a balance of procoagulants (eg, FVIII, FIX) and the natural anticoagulants (Figure 1). A reduction in any of the procoagulants leads to a bleeding phenotype. Restoration of hemostatic balance can be achieved by replacement of the missing procoagulant. However, several lines of evidence have demonstrated that the hemostatic balance may also be restored by inhibiting/reducing the natural anticoagulants even without replacing the missing procoagulant.26 Clinical evidence has been provided by the observation that coinheritance of thrombophilic risk factors can moderate the clinical phenotype of severe hemophilia A.27,28 Thrombin generation is increased in the plasma of individuals with coinheritance of hemophilia and some thrombophilias (eg, protein C deficiency).29 In addition, reducing antithrombin levels enhances the hemostatic responses to recombinant VIIa in vitro in FVIII-deficient plasma30 and in vivo in a murine hemophilia A model.31 Pipeline programs are now investigating targeting each of the natural anticoagulants as alternative therapeutics for hemophilia.
In 2006, Fire and Mello won the Nobel Prize for discovery of short interfering RNA (siRNA) regulation of gene expression.32 The ability to decrease or eliminate expression of specific genes had obvious clinical and therapeutic applications, but the delivery of siRNA to target cells proved challenging.33 The development of technologies that permitted reliable delivery of functional siRNA to hepatocytes opened the door to therapeutics targeting proteins produced in the liver.34 Antithrombin (AT) is a small protein member of the serpin family, produced in hepatocytes, and is a potent inhibitor of thrombin. Because thrombin is central to coagulation and leads to thrombus formation when in stoichiometric excess of AT, its most important antagonist, hepatocyte siRNA silencing of AT RNA and resultant reduction in plasma AT levels is an attractive approach to treatment of bleeding disorders. Complete absence of AT is not compatible with life; significantly reduced levels have been associated with pathologic thrombus formation. In mice treated with siRNA against AT, life was shortened secondary to thrombotic disease. However, in hemophilia mice, siRNA targeting of AT resulted in prolonged life through protection from bleeding.35 GalNAC-AT3 siRNA (fitusiran) were able to enter hepatocytes and reliably reduce AT plasma levels, increase thrombin generation, and reduce bleeding in a dose-dependent fashion.35 Recent results from a phase 1 dose escalation study of fitusiran in 4 healthy volunteers and 25 participants with hemophilia demonstrated consistent dose dependent reduction in AT with weekly or monthly subcutaneous dosing; with a standard dose of 80 mg subcutaneously administered monthly, there was a 70% to 89% reduction in AT.36 This resulted in improved thrombin generation. Mild elevations of D-dimer were seen and 9 subjects had mild, asymptomatic elevations in transaminases, 8 of whom had hepatitis C and did not exceed 3 times the upper limit of normal. A phase 2 open label extension study is investigating the safety and efficacy of 50 mg and 80 mg of fitusiran in subjects with hemophilia A and B, with and without inhibitors. Interim data have shown that the overall median ABR in all patients was 1, with 48% (16/33) of subjects bleeding free during the observation period and 67% (22/33) of subjects reporting no spontaneous bleeds.37 In September 2017, the phase 2 open label extension study was temporarily closed after a fatal severe adverse reaction. A subject on the study with hemophilia A developed hip pain after exercise and treated the bleed with typical doses of FVIII. He subsequently developed a headache and a computed tomography scan of the head was initially interpreted to demonstrate a central nervous system bleed prompting more intense FVIII replacement. The subject developed cerebral edema and died. Subsequent interpretation of the computed tomography scan of the head revealed that the subject had suffered a cerebral venous sinus thrombosis and not central nervous system bleeding. The phase 2 study was reopened after a mitigation strategy was put in place including education strategies and protocol specified reduced dosing of FVIII or bypass agents for the treatment of breakthrough bleeding. This drug has attractive features including subcutaneous route, infrequent dosing (once a month), and similar hemostatic improvement in hemophilia A and B.
Another approach to altering hemostatic balance is interference with TFPI-mediated inhibition of the extrinsic pathway. TFPIα is the soluble isoform that is responsible for the inhibition of the prothrombinase activity of the extrinsic clotting system with a K1 domain that inhibits FVIIa, a K2 domain that inhibits FXa, and a K3 domain that binds protein S.38,39 Hemophilia mice with TFPI knocked out in hematopoietic stem cells are protected from bleeding,40 and inhibition of TFPI reduced bleeding in several hemophilia animal models.41-43 Aptamer,42 fucoidan,43 monoclonal antibody,41 and peptide44 agents have been developed to inhibit TFPI. An aptamer inhibitor of TFPIα K1 and K3 domains45 was studied in humans but resulted in increased plasma TFPIα and increased bleeding.46 A phase 1 trial of concizumab, a monoclonal antibody directed at the K2 domain, in healthy volunteers and people with hemophilia demonstrated dose-dependent increases in D-dimer and prothrombin fragments 1 and 2 as well as a favorable safety profile and half-life whether given intravenously or subcutaneously.47 Several pharmaceutical manufacturers are now pursuing TFPI as a target for new therapies for hemophilia with inhibitors and other bleeding disorders.
Inhibition of activated protein C (APC) is the most recent target for a novel therapeutic for bleeding disorders. The principle is to inhibit activated protein C whose action results in proteolytic degradation of FVa with resultant dampening of the intrinsic pathway amplification. Endogenous inhibitors of protein C include protein C inhibitor and α1-antitrypsin; however, these inhibitors both suffer from poor reactivity and selectivity toward APC. APC-binding aptamers have been recently described which can selectively block the anticoagulant functions of APC.48 Polderdijk et al have now described an α1-antitrypsin variant in which they introduced mutations in and around the reactive center P1-P1’ bond of α1-antitrypsin.49 This resulted in enhanced specificity for APC without inhibiting thrombin, FXa or other relevant coagulation proteases. Notably, this molecule rescued thrombin generation in the presence of thrombomodulin and was found to restore hemostasis in hemophilia mice. With a subcutaneous route of administration and a long half-life, this molecule could also be developed as an alternative prophylaxis agent for hemophilia.
Improved inhibitor eradication and prevention
One of the biggest challenges of the current therapeutic approach has been the development of neutralizing antibodies to replacement factor in ∼3% of people with hemophilia B and 30% of people with hemophilia A. Inhibitor allo-antibodies result in significant increase in morbidity and mortality50 for persons with hemophilia and render recombinant or plasma-derived factor replacement ineffective, requiring use of bypassing coagulation agents such as recombinant FVIIa and activated prothrombin complexes. These alternative therapeutics result in an exponentially higher cost51 and administration burden despite inconsistent hemostatic efficacy. The standard of care for people with inhibitors to FVIII has been immune tolerance induction using high daily doses of replacement FVIII that achieves eradication of the inhibitor antibodies and tolerance to FVIII ∼70% of the time. Although the mechanism underlying tolerance induction is incompletely understood in hemophilia, we have increasingly been able to identify factors associated with increased inhibitor development including large gene deletions, family history, race/ethnicity, age, and intensity of first exposure to factor and factor product source (eg, plasma-derived vs recombinant).52,53
The Survey of Inhibitors in Plasma-Products Exposed Toddlers trial randomized previously untreated hemophilia A patients (PUPs) to treatment with a plasma-derived or recombinant FVIII product and demonstrated an 87% lower incidence of inhibitors in the PUP treated with plasma-derived products.52 The mechanism of plasma product protection from inhibitor formation remains unclear. The Survey of Inhibitors in Plasma-Products Exposed Toddlers trial, however, compared only standard half-life second- and third-generation recombinant products and did not include newer recombinant products such as those produced in human cell lines or that have been structurally modified for extended half-life (Fc and albumin fusion or polyethylene glycol [PEG] conjugates). These newer recombinant products may be potentially less immunogenic by several mechanisms: (1) elimination of nonhuman glycans (human cell line production), (2) increased exposure time after infusion, and (3) shielding of epitopes from the immune system. PEG conjugates have been used previously to circumvent some immune responses.54 Fc fusion may also act as an immunomodulatory agent by increasing T-regulatory cell response.55 The incidence of inhibitors within PUPs with these therapeutics is under investigation as are studies that will investigate their efficacy in immune tolerance induction (Table 2). Although these approaches may provide incremental improvement in inhibitor incidence and eradication, inhibitor formation could be avoided entirely through complete avoidance of exposure to FVIII or FIX, as may now be possible with the availability of emicizumab or those agents that target the natural anticoagulants.
Trials examining role of Fc fusion or PEGylation in prevention and eradication of inhibitors
| Trial . | Population . | Product . | Outcome . |
|---|---|---|---|
| INHIBIT | Previously untreated hemophilia A patients | Fc-rFVIII | Inhibitor formation T-regulatory cells |
| Adynovate PUP | Previously untreated hemophilia A patients | PEG-rFVIII | Inhibitor formation |
| VerITI-8 | Hemophilia A patients with high titer inhibitors | Fc-rFVIII | Induction of tolerance |
| Trial . | Population . | Product . | Outcome . |
|---|---|---|---|
| INHIBIT | Previously untreated hemophilia A patients | Fc-rFVIII | Inhibitor formation T-regulatory cells |
| Adynovate PUP | Previously untreated hemophilia A patients | PEG-rFVIII | Inhibitor formation |
| VerITI-8 | Hemophilia A patients with high titer inhibitors | Fc-rFVIII | Induction of tolerance |
INHIBIT, Hemophilia Inhibitor Prevention Trial; VerITI-8, A Study to Evaluate Efficacy of rFVIIIFc for Immune Tolerance Induction (ITI) in Severe Hemophilia A Participants With Inhibitors Undergoing the First ITI Treatment.
Recombinant VWF
Since the description of the index case of VWD in 1926, there has been a dramatic increase in our knowledge of the diagnosis and management of the most common bleeding disorder in humans. Although expertly reviewed elsewhere,56 we will provide a brief synopsis of the biosynthesis of VWF to provide context for the subsequent discussion on the individualized treatment of VWD with a focus on a new product, Vonvendi, the first recombinant VWF (rVWF) product.
In VWD, the mainstay of treatment is replacement of defective or deficient VWF to promote hemostasis in prevention or treatment of bleeding. A variety of virally inactivated intermediate and high purity plasma-derived concentrates (pdVWFs) are available on the market. However, until late 2015, there were no available rVWF products because of complex structure, multiple posttranslational steps, and large size.57
rVWF production is independent of the blood supply and has an optimized ratio of VWF:ristocetin cofactor activity (RCo) to FVIII for treatment of patients with severe VWD to mitigate risk of FVIII accumulation with multiple doses and decrease risk of thrombosis.58 A comprehensive preclinical program examined the safety and efficacy of rVWF (Table 3).
Summary of select differences between human VWF, VWF/FVIII concentrates, and rVWF
| Characteristics of rVWF . | Comments . |
|---|---|
| Carbohydrate structure/glycosylation pattern | Similar to human platelet VWF and lacking ABO antigens |
| Ultra large multimers | Intact UL-HMW from lack of ADAMTS13 exposure in processing before infusion |
| Multimer triplet pattern | Intact multimer pattern from lack of exposure to ADAMTS13 during processing, forms triplet pattern in vivo |
| Performance in VWF functional assays | Functional activity of rVWF similar with regard to VWF:FVIIIB but higher in VWF:RCo and VWF:CB and VWF:RCo/VWF:Ag >1 indicating higher VWF specific activity |
| Platelet adhesion/aggregation assay results | No evidence of exaggerated or activation independent platelet binding despite presence of UL-HMW multimers. Reduced closure times consistently of PFA-100 compared with pdVWF |
| Characteristics of rVWF . | Comments . |
|---|---|
| Carbohydrate structure/glycosylation pattern | Similar to human platelet VWF and lacking ABO antigens |
| Ultra large multimers | Intact UL-HMW from lack of ADAMTS13 exposure in processing before infusion |
| Multimer triplet pattern | Intact multimer pattern from lack of exposure to ADAMTS13 during processing, forms triplet pattern in vivo |
| Performance in VWF functional assays | Functional activity of rVWF similar with regard to VWF:FVIIIB but higher in VWF:RCo and VWF:CB and VWF:RCo/VWF:Ag >1 indicating higher VWF specific activity |
| Platelet adhesion/aggregation assay results | No evidence of exaggerated or activation independent platelet binding despite presence of UL-HMW multimers. Reduced closure times consistently of PFA-100 compared with pdVWF |
A phase 1 multicenter randomized clinical trial evaluated the pharmacokinetics and safety of rVWF in 32 adult patients with genetically confirmed severe type 1 or type 3 VWD.59 Vonvendi had a slightly longer half-life compared with pdVWF as measured by VWF:RCo (16.3 vs 14.4 hours) and by VWF:antigen (25.5 vs 17.9 hours). No serious adverse events or thrombotic complications were noted. Furthermore, rVWF could be given alone after a therapeutic FVIII activity was achieved with the initial dose. In the subsequent phase 3 trial, which assessed efficacy, safety, and pharmacokinetics, rVWF was evaluated in comparison with rVWF + rFVIII in a crossover design.60 Interestingly, the pharmacokinetic profile was not different with the addition of rFVIII to rVWF. Further, FVIII activity increased in 6 hours following infusion of rVWF alone and was subsequently sustained over the next 72 hours, indicating optimal stabilization of endogenous FVIII. More than 96% of bleeds were reported to have had excellent response after a single infusion. Although differing rating systems and doses were used, >80% of bleeds were treatable with a single infusion. Finally, safety was defined with no thrombotic events, serious adverse events, allergic reactions, VWF or FVIII inhibitors, or anti-VWF binding or host cell antibodies detected.
The high purity, higher functional VWF activity and intact multimer pattern should be considered in bleeding situations in which previous studies have shown limited hemostatic efficacy from pdVWF concentrates, such as control of gastrointestinal bleeding in patients with VWD with angiodysplasia. rVWF may also be beneficial in patients with VWD and a baseline elevated FVIII activity or in people with severe VWD on prophylaxis, because immediate normalization of FVIII is not critical. Contrarily, in surgical situations in which the FVIII activity is <40% and immediate surgery is needed, a pdVWF concentrate may be optimal economically in comparison with the use of both a rFVIII and rVWF. Similarly, for acute major bleeds in patients with FVIII <40%, a pdVWF concentrate may be more practical. The use of rVWF is likely to be refined over time with additional cumulative experience such as in the management of patients with type 2N VWD.61
Conclusions
Decades of investigation have advanced our knowledge of the hemostatic system, viral gene therapy, and many other technologies leading to recent advancements in therapy for bleeding disorders. These new therapies offer many options for improved treatment and even cures of these disorders, but will require ongoing study to determine their optimal use and ensure safety.
Authorship
Contribution: M.U.C., R.S., and S.W.P. contributed equally to the concept, writing, and editing of this review.
Conflict-of-interest disclosure: M.U.C. has participated in advisory boards and received honoraria from Shire, Octapharma, Grifols, Pfizer, Bayer, and Roche/Genentech; been on paid speaker bureaus for Shire and Roche/Genentech; received research support from Shire and Pfizer; been a site investigator or subinvestigator of clinical trials for Pfizer, Roche/Genentech, Novo Nordisk, Global Blood therapeutics, Sancillio, and Amgen; and owns stock in Alnylum. R.S. has had research funding from Shire, Grifols, and Bioverativ and received payment for consulting for Shire, Novo Nordisk, CSL Behring, Bioverativ, Bayer, and Pfizer. S.W.P. has received research funding from Shire and Siemens and has received payment for consulting with Shire, Novo Nordisk, CSL Behring, Bioverativ, Bayer, Pfizer, uniQure, Dimension Therapeutics, and Biomarin.
Correspondence: Steven W. Pipe, Departments of Pediatrics and Pathology, University of Michigan, 1500 E Medical Center Dr, Room L2110, Ann Arbor, MI 48109-0237; e-mail: ummdswp@med.umich.edu.