In this issue of Blood, Ward et al demonstrate that a loss of sialic acids turns von Willebrand factor (VWF) into a ligand for the scavenger-receptor macrophage galactose-type lectin (MGL).1
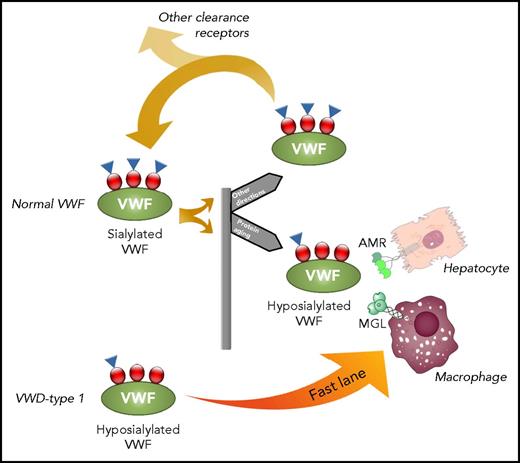
The role of MGL in the clearance of VWF. VWF contains glycan structures (red dots) that are capped by sialic acids (blue triangles). Circulating VWF may lose some of the terminal sialic acids over time and becomes hyposialylated. Hyposialylated VWF is a target for the AMR on hepatocytes. However, in this issue, Ward and colleagues now show that MGL is also an active receptor for hyposialylated VWF, mediating rapid macrophage-mediated clearance. Interestingly, van Schooten et al5 reported previously that type 1 VWD is often associated with reduced sialylation of O-linked glycans and concomitant increased clearance (fast lane). It is therefore tempting to speculate that MGL also contributes to the increased clearance of such hyposialylated VWF in type 1 VWD.
The role of MGL in the clearance of VWF. VWF contains glycan structures (red dots) that are capped by sialic acids (blue triangles). Circulating VWF may lose some of the terminal sialic acids over time and becomes hyposialylated. Hyposialylated VWF is a target for the AMR on hepatocytes. However, in this issue, Ward and colleagues now show that MGL is also an active receptor for hyposialylated VWF, mediating rapid macrophage-mediated clearance. Interestingly, van Schooten et al5 reported previously that type 1 VWD is often associated with reduced sialylation of O-linked glycans and concomitant increased clearance (fast lane). It is therefore tempting to speculate that MGL also contributes to the increased clearance of such hyposialylated VWF in type 1 VWD.
VWF is a protein that is mostly known for its involvement in the hemostatic pathway, and its deficiency is associated with severe bleeding complications in a disorder known as von Willebrand disease (VWD). In the early 2000s, it was found that abnormal clearance of VWF contributes to the pathogenesis of VWD. Together with the notion that VWF regulates clearance of coagulation factor VIII (FVIII; which is highly relevant for treatment of hemophilia A), studies on VWF clearance have therefore drawn increasing attention.2 In particular, the role of VWF glycosylation has been of particular interest for 2 reasons. First, we know that ABO-blood group glycan structures are a major determinant of VWF plasma levels. Second, in the 1970s, it had already been found that the presence of sialic acids was pertinent to the in vivo survival of VWF, a finding that has since been confirmed in several other studies.3-5
Loss of sialic acids generates hyposialylated VWF, in which exposed galactose residues can be recognized by several lectin-like receptors, with the Ashwell-Morell receptor (AMR) being their quintessential representative. The active participation of the AMR in eliminating hyposialylated VWF has been shown in an elegant study by Grewal et al.6 However, in the present study, Ward et al noticed that deficiency of the AMR was insufficient to correct increased clearance of hyposialylated VWF, irrespective of whether all sialic acids were removed or only those on O-linked glycans. This led them to conclude that besides the AMR, other lectin receptors should also contribute to the removal of hyposialylated VWF. In their search for alternative receptors, Ward et al found that chemical depletion of macrophages markedly enhanced survival of hyposialylated VWF, pointing to a macrophage-specific receptor. They further focused on a lectin-like receptor named MGL, which is expressed on macrophages and dendritic cells. Several lines of evidence confirmed that MGL plays an active role in removing hyposialylated VWF from the circulation.1 For instance, the presence of anti-MGL antibodies impairs clearance of sialidase-treated VWF in AMR-deficient mice. Furthermore, basal VWF levels are significantly increased in mice lacking the murine ortholog of human MGL (Mgl1−/− mice). Finally, clearance of VWF was delayed in Mgl1−/− mice.1
Having established MGL as a novel clearance receptor for VWF, the next step would be to define its relative contribution in relation to the spectrum of other VWF receptors previously described. Ward et al first refer to the recent description of an intrinsic pathway of protein aging and turnover in which circulating proteins are constantly subjected to the activity of glycosidases, including sialidases that remove sialic acids.7 Thus, the longer a protein circulates in plasma, the more hyposialylated it will become. In the case of VWF, this would mean that hyposialylated VWF becomes a target for MGL, contributing to the removal of aged VWF from the circulation (see figure). As such, MGL would be different from other macrophage receptors, like LRP1 (known to interact with VWF in a shear stress–dependent manner) and scavenger-receptor AI (recently reported to contribute to basal VWF clearance).8,9 The data presented by Ward et al further indicate that MGL would have a larger contribution to the clearance of hyposialylated VWF as compared with the AMR.
A final point of interest relates to the increased clearance of VWF that has been observed in VWD-type 1. Recent studies showed that specific mutations may increase the binding of such VWF mutants to LRP1 and/or scavenger-receptor AI.9,10 In addition, another study also showed that many VWD-type 1 mutations are associated with reduced sialylation of O-linked glycan structures.5 In light of the report by Ward et al, it now seems conceivable that the increased clearance observed in VWD-type 1 patients can originate from premature binding of these mutants to MGL, due to hyposialylation of the O-linked glycan structures (see figure fast lane). In this regard, it would be of interest to investigate whether polymorphisms in the gene encoding MGL are associated with modified VWF levels, particularly in VWD-type 1 patients. Another relevant avenue to explore would be the role of MGL in the clearance of FVIII. Is MGL-mediated clearance limited to VWF, or does it also include the VWF/FVIII complex? And if so, would making VWF resistant to desialylation improve its half-life and that of FVIII? Upon further studies on these matters, it is without doubt that MGL is another player in the complicated pathway of VWF clearance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.