Key Points
Blinatumomab delays deterioration in HRQL in adults with R/R ALL.
Abstract
In the phase 3 TOWER study, blinatumomab significantly improved overall survival in adults with relapsed or refractory (R/R) Philadelphia chromosome–negative (Ph−) B-cell precursor acute lymphoblastic leukemia (BCP-ALL) relative to standard-of-care chemotherapy. A secondary objective of this study was to assess the impact of blinatumomab on health-related quality of life (HRQL) as measured by the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30). This analysis included the 342 of 405 randomized patients for whom baseline and ≥1 postbaseline result were available in any EORTC multi-item scale or single-item measure. In general, patients receiving blinatumomab (n = 247) reported better posttreatment HRQL across all QLQ-C30 subscales, based on descriptive mean change from baseline, than did those receiving chemotherapy (n = 95). The hazard ratios for time to deterioration (TTD) of ≥10 points from baseline in HRQL or death ranged from 0.42 to 0.81 in favor of blinatumomab, with the upper bounds of the 95% confidence interval <1.0 across all measures, except insomnia, social functioning, and financial difficulties; sensitivity analysis of TTD in HRQL without the event of death were consistent with these findings. When treatment effect over time was tested using a restricted maximum likelihood–based mixed model for repeated measures analysis, P < .05 was reached for blinatumomab vs chemotherapy for all subscale measures except financial difficulties. The clinically meaningful benefits in overall survival and HRQL support the clinical value of blinatumomab in patients with R/R Ph− BCP-ALL when compared with chemotherapy. This trial was registered at www.clinicaltrials.gov as #NCT02013167.
Introduction
The prognosis is poor for patients with acute lymphoblastic leukemia (ALL) who relapse or are refractory to treatment.1-4 The approval of blinatumomab (Blincyto) by the US Food and Drug Administration (FDA) in 2014 provided an innovative treatment option for patients with relapsed or refractory (R/R) Philadelphia chromosome–negative (Ph−) B-cell precursor (BCP)-ALL. Blinatumomab is a bispecific immunotherapy directed against CD19 and CD3. In a phase 3 study of patients with R/R Ph− BCP-ALL (TOWER), blinatumomab was shown to nearly double median overall survival compared with the standard-of-care salvage chemotherapy group (7.7 months vs 4.0 months); complete remission rates, event-free survival, duration of remission, and depth of remission were all improved with blinatumomab compared with standard chemotherapy.5
The TOWER study also confirmed an acceptable toxicity profile for blinatumomab. Essentially all patients (99%) experienced adverse events, and most patients experienced adverse events of grade 3 or higher (blinatumomab, 87%; chemotherapy, 92%). The majority of adverse events resolved during the assessment period. Neutropenia and infection were the most common grade 3 or higher adverse events in both groups. Neutropenia of grade 3 or higher occurred in 37.8% of patients in the blinatumomab group and in 57.8% of patients in the chemotherapy group, whereas grade 3 or higher infection occurred in 34.1% and 52.3% of patients, respectively.5 The majority of neurotoxic events, cytokine release syndrome events, and infections resolved during the assessment period.
To complement these efficacy and safety analyses, and in view of the intensive nature of conventional treatment in R/R ALL, patient-reported outcomes (PROs) were also assessed to determine the impact of blinatumomab on health-related quality of life (HRQL) compared with salvage chemotherapy. Quality-of-life (QoL) considerations are important determinants in defining how treatments are used in clinical practice as they provide not only a measure of clinical effectiveness, but also gauge the value of the treatment in a way that is uniquely patient centric.6-8 HRQL data in patients with R/R ALL are surprisingly limited with the INO-VATE trial being the only other phase 3 study in this patient population reporting HRQL results.7,9 In the INO-VATE study of inotuzumab ozogamicin vs standard-of-care chemotherapy (SOC), complete remission rates were significantly improved with inotuzumab ozogamicin although overall survival was not statistically significantly longer10 ; PROs were generally favorable for inotuzumab ozogamicin compared with SOC.9 The primary aim of this report is to describe the HRQL outcomes of the TOWER study.
Methods
Study design
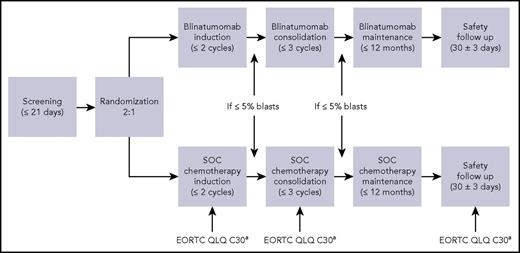
TOWER was a prospective, multicenter, open-label, randomized, controlled phase 3 study designed to compare blinatumomab with SOC in patients with R/R Ph− BCP-ALL. Patients were randomized 2:1 to receive blinatumomab or chemotherapy. Randomization was stratified by age, previous salvage therapy, and previous allogeneic hematopoietic stem cell transplantation (alloHSCT). All patients provided written informed consent. The trial protocol was approved by the investigational review board or independent ethics committee at each trial center. Study design details are described elsewhere.5
Study population
Patients ≥18 years old with Ph− BCP-ALL who were refractory to primary induction therapy or refractory to salvage therapy, in untreated first relapse with first remission duration <12 months, in untreated second or greater relapse, or in relapse at any time after alloHSCT were eligible to participate. Patients with a history of malignancy other than ALL or with active ALL in the central nervous system were excluded. Study population details are described elsewhere.5
Treatment protocol
Blinatumomab was administered as a continuous IV infusion in a 6-week cycle, which included 4 weeks of blinatumomab followed by a 2-week treatment-free interval. Overall, blinatumomab treatment consisted of 2 induction cycles, up to 3 consolidation cycles, and up to an additional 12 months of maintenance therapy. Patients were recommended to be hospitalized during the first 9 days of cycle 1 and the first 2 days of subsequent cycles. The actual hospitalization length of stay was dependent on the investigator’s judgment and tolerability of blinatumomab, although hospitalization for at least the first 2 days was required in cycle 1 and cycle 2 as well as after any increase in dose. In cycle 1, blinatumomab was dosed at 9 µg per day for 7 days, and then the dose was increased to 28 µg per day on days 8 through 29, and for all subsequent cycles.
Patients randomized to chemotherapy were assigned by investigator’s choice to receive 1 of 4 chemotherapy regimens: fludarabine, high-dose cytarabine, granulocyte colony-stimulating factor (FLAG) plus or minus an anthracycline-based regimen; a high-dose cytarabine (HiDAC)-based regimen with cytarabine arabinoside plus or minus anthracycline plus or minus other drugs; a high-dose methotrexate-based regimen in combination with other drugs; or clofarabine or clofarabine-based regimens.5 The duration of chemotherapy was left to the investigator (Figure 1).
TOWER schematic of HRQL assessment schedule. An EORTC QLQ-C30 was completed on day 1, day 8 (cycle 1 only), day 15, day 29 ± 8 days of each cycle and at the safety follow-up visit.
TOWER schematic of HRQL assessment schedule. An EORTC QLQ-C30 was completed on day 1, day 8 (cycle 1 only), day 15, day 29 ± 8 days of each cycle and at the safety follow-up visit.
Methods of HRQL assessment
To assess HRQL, patients completed the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30) via electronic data capture on day 1 (baseline), day 8 (cycle 1 only), day 15, and day 29 of each cycle of investigational therapy and on the safety follow-up visit (Figure 1). The self-administered questionnaire was to be completed before any other clinical assessments and before receiving any study medications. Patients could have the questions read to them if they were blind or illiterate, but study staff could not interpret any of the questions for the patient.
The EORTC QLQ-C30 is a 30-item validated self-rating questionnaire for assessing the HRQL of patients with cancer participating in clinical trials; it is composed of 15 subscales (5 functional scales [physical, role, emotional, cognitive and social], 3 symptom scales [fatigue, nausea and vomiting, pain], global health status/QoL [GHS/QoL], and 6 single items [dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties]).11 A linear transformation was used to standardize the raw scores, so each domain was scored from 0 to 100. Higher GHS/QoL, function, or symptoms scores indicate better HRQL, better functioning, or more severe symptoms, respectively.
Statistical analyses
A prespecified secondary end point for clinical relevance in the TOWER study was the time to a ≥10-point decrease from baseline in GHS/QoL or death, whichever came first. Prespecified exploratory end points included time to a ≥10-point deterioration from baseline or death for each individual subscale measured in EORTC QLQ-C30, and the change from baseline in EORTC QLQ-C30 scales/items.
Analysis of HRQL was based on the reported change in each EORTC QLQ-C30 scale scores relative to baseline. The analysis set consisted of patients with baseline (day 1 before the initiation of protocol-specified therapy) and ≥1 postbaseline result of any EORTC QLQ-C30 multi-item or single-item scale measure. Scores were derived using the sum of the responses from all of the associated questions and standardized to the range 0 to 100.11 For the multi-item scales with answers for at least half of the items, missing responses were imputed based on the average of those items answered; if more than half of the responses were missing, the score was classified as missing.
The mean (standard deviation [SD]) changes from baseline by visit and by treatment group were summarized for each of the 15 scales of the EORTC QLQ-C30 evaluation. The mean (SD) changes from baseline by visit and by treatment group, and response status, were also summarized.
For each EORTC QLQ-C30 scale, the time to deterioration (TTD) in HRQL or death, whichever came first, was compared between the blinatumomab and chemotherapy groups.12-15 For the GHS/QoL and function subscales, response was defined as ≥10-point change from baseline: deterioration was defined as a ≥10-point decrease from baseline; for symptom subscales, deterioration was defined as a ≥10-point increase from baseline.16 Time was calculated from baseline to deterioration or death, whichever came first. Patients who did not have an event were censored at the time of their last EORTC QLQ-C30 assessment. A second analysis was conducted that evaluated TTD in HRQL, without the event of death, for each EORTC QLQ-C30 scale. The TTD and TTD or death were compared between the blinatumomab and chemotherapy groups using a 2-sided stratified log-rank test, stratified by randomization factors. In addition, a hazard ratio (HR) with a 95% confidence interval (CI) was estimated from a stratified Cox regression model. Kaplan-Meier summaries were performed by treatment group. Significance testing was considered descriptive. All statistical tests were 2-sided with a P value <.05 used to identify significance; P values were not adjusted for multiplicity. All analyses were performed using SAS statistical software version 9.4 or higher.
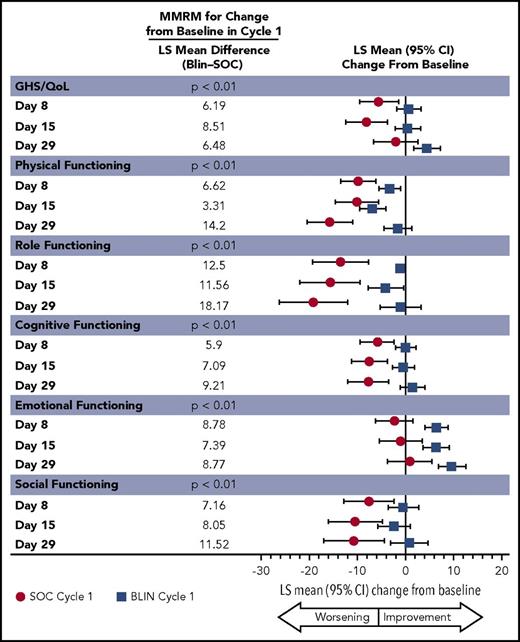
To estimate the treatment effect over time, changes from baseline in each EORTC QLQ-C30 scale score were compared between treatment groups using a restricted maximum likelihood–based mixed model for repeated measures (MMRM) analysis under the assumption of missing at random (MAR) in cycle 1, which had low percentages of unexplained missing entries. As there was no way to check whether missingness was independent of unobserved variables, the MAR assumption was not validated.17 Multiple imputation sensitivity analyses for MAR assumption were conducted via R package “mice.”18 Missing scores in each HRQL scale during first cycle visits were imputed 10 times using a mixed model of patient’s treatment, time, and known scores. Least-squares (LS) means of change from baseline were calculated.
The dependent variable of this model was the change from baseline EORTC QLQ-C30 scale scores measured at days 8, 15, and 29 of cycle 1. Data from cycle 1 were used as subsequent cycles had too few patients for meaningful results. The adjusted average difference from baseline for each time point was estimated using LS means from the MMRM model. The MMRM included the effects of treatment, visit, treatment by visit interaction, and baseline score. The LS mean treatment difference used to detect a clinically meaningful difference between blinatumomab and chemotherapy was 5 points.19,20
The reporting of these PRO data are consistent with the CONSORT PRO recommendations.21
Results
Patient population
Between January 2014 and September 2015, 405 patients from 101 centers in 21 countries were randomized (271 blinatumomab; 134 chemotherapy). The HRQL data came from 96 of 101 centers. Of the 5 centers without HRQL data (6 patients), 3 centers had patients randomized but not treated (3 patients). Median age was 37 years, 59% were male, and 84% were white; 55.8% had prior salvage therapy and 34.6% had prior alloHSCT. The baseline characteristics were well balanced between the 2 treatment groups. Of the 376 patients who received ≥1 dose of study drug, 342 patients (247 blinatumomab, 95 chemotherapy) had pretreatment EORTC QLQ-C30 baseline scores and ≥1 postbaseline response. Patients in the blinatumomab and chemotherapy groups answered an average of 94.1% and 93.5% of subscale measures, respectively. Mean pretreatment baseline EORTC QLQ-C30 scores were balanced between the blinatumomab and chemotherapy groups; baseline demographics and characteristics were balanced between groups in the EORTC QLQ-C30 population and comparable to the intent-to-treat (ITT) population (supplemental Table 1, available on the Blood Web site). Overall, the questionnaire completion rates among available patients (ie, those surviving) were high in cycle 1, especially given the severity of their condition, and were slightly higher for the blinatumomab group. Cycle 1 completion rates ranged from 72% to 89% for the blinatumomab group and 60% to 85% for the chemotherapy group (supplemental Table 2). Completion rates for subscales were higher. For example, completion rates for GHS/QoL for blinatumomab ranged from 94% (day 1, cycle 1) to 79% (day 29, cycle 1); for SOC, completion rates ranged from 94% (day 1, cycle 1) to 70% (day 29, cycle 1). Most patients who discontinued SOC or blinatumomab following cycle 1 did so as a result of disease progression or to receive additional therapy/alloHSCT (supplemental Table 3). The number of patients continuing beyond cycle 1 in the EORTC QLQ-C30 population was too small for meaningful HRQL analyses.
Efficacy
As reported previously, overall survival for blinatumomab was improved significantly compared with chemotherapy at the time of interim analysis (7.7 vs 4.0 months), as was complete remission with full (CR), partial (CRh), or incomplete (CRi) hematologic recovery (44% vs 25%; P < .001) after 12 weeks for the ITT population.5 The study was discontinued prematurely for benefit in the blinatumomab group after the second interim analysis (when 75% of the total number of deaths had occurred) on the recommendation of the data monitoring committee because the P value was less than the prespecified threshold P value for overall survival. By the data cutoff date, 68.9% of patients had discontinued treatment. The most common reason for study discontinuation was death (60.5%).
In the EORTC QLQ-C30 analysis set after cycle 1 (6 weeks), CR/CRh/CRi was achieved in 34% of patients treated with blinatumomab (85 of 247) and 34% of patients treated with chemotherapy (32 of 95) . At the end of cycle 1, 36 patients had died (8.9% [22/247] blinatumomab; 14.7% [14/95] chemotherapy) in the EORTC QLQ-C30 analysis set.
Longitudinal changes in EORTC QLQ-C30 scores
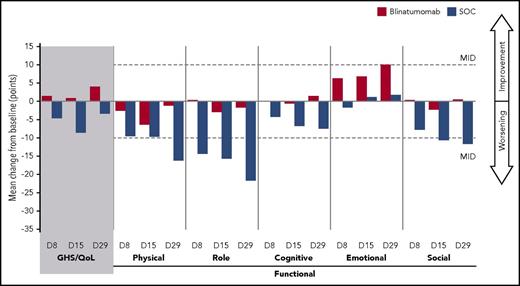
In the blinatumomab group, mean changes from baseline in GHS/QoL, functional scales, and symptom scales were minimal across cycle 1 (Figures 2 and 3). In contrast, in the chemotherapy group, a worsening in EORTC QLQ-C30 scores was apparent, with mean changes near or exceeding the 10-point threshold for deterioration in about half of the scale scores. The deterioration in chemotherapy EORTC QLQ-C30 scale scores was especially apparent for chemotherapy nonresponders (supplemental Figures 1 and 2) vs chemotherapy responders (supplemental Figures 3 and 4). At some point during cycle 1, a ≥10-point mean decrease in GHS/QoL and physical, role, cognitive, and social function; and a ≥10-point mean increase in symptoms of fatigue, pain, nausea and vomiting, dyspnea, appetite loss, constipation, and diarrhea were observed for chemotherapy nonresponders whereas no ≥10-point mean detriment in any scale was observed for blinatumomab nonresponders (supplemental Figures 1 and 2). The observed trends in EORTC QLQ-C30 scores for both treatment groups in cycle 1 were also observed in cycle 2, although the number of patients remaining in the chemotherapy group was small (n = 27 for cycle 2, day 1; n = 15 for cycle 2, day 29).
Global health status and functional scale: cycle 1 all subjects. EORTC QLQ-C30 analysis set: n = 247 blinatumomab, n = 95 SOC. D, day.
Global health status and functional scale: cycle 1 all subjects. EORTC QLQ-C30 analysis set: n = 247 blinatumomab, n = 95 SOC. D, day.
Symptoms: cycle 1 all subjects. EORTC QLQ-C30 analysis set: n = 247 blinatumomab, n = 95 SOC.
Symptoms: cycle 1 all subjects. EORTC QLQ-C30 analysis set: n = 247 blinatumomab, n = 95 SOC.
TTD in HRQL or death
The time to clinically meaningful deterioration in HRQL or death was delayed for blinatumomab vs chemotherapy across all EORTC QLQ-C30 scales. A longer TTD in HRQL or death was observed for patients treated with blinatumomab vs chemotherapy (HR < 1.0; P < .05) for all functional scales (except social functioning) and for all symptom scales (except insomnia and financial difficulties). The forest plot in Figure 4A depicts the TTD in HRQL or death for all of the EORTC QLQ-C30 scales. Specifically, for GHS/QoL, the median time (95% CI) to ≥10-point deterioration or death was 1.7 (1.1, 3.6) months for the blinatumomab group vs 1.0 (0.5, 1.5) months for the chemotherapy group (HR, 0.66 [0.48, 0.92]; P = .009) (supplemental Figure 5).
Time to clinically meaningful deterioration in HRQL or death. (A) Time to clinically meaningful deterioration in HRQL or death analyses. (B) Time to clinically meaningful deterioration in HRQL only. aStratified HR for EORTC QLQ-C30 for treatment difference. bStratified log-rank test for EORTC QLQ-C30 for treatment difference. EFS, event-free survival.
Time to clinically meaningful deterioration in HRQL or death. (A) Time to clinically meaningful deterioration in HRQL or death analyses. (B) Time to clinically meaningful deterioration in HRQL only. aStratified HR for EORTC QLQ-C30 for treatment difference. bStratified log-rank test for EORTC QLQ-C30 for treatment difference. EFS, event-free survival.
The TTD in HRQL without the event of death analysis showed similar delays in deterioration for the blinatumomab group compared with the chemotherapy group across all of the scales in EORTC QLQ-C30 (Figure 4B; supplemental Figure 6) as did the TTD in HRQL or event-free survival analysis (supplemental Figure 7).
Treatment group differences
The between-group treatment effect for the change from baseline in cycle 1 as determined by the MMRM was consistent with the descriptive analyses, with P < .05 favoring blinatumomab for all EORTC QLQ-C30 subscales except financial difficulties, for which there was no difference (Figures 5 and 6). The least squares mean treatment differences between blinatumomab and chemotherapy were ≥5 points across the majority of subscales, suggesting clinically important differences between the 2 treatments.19,20 Pooled results from multiple imputation sensitivity analyses for MAR assumption show similar directions and magnitudes of LS means of change from baseline (supplemental Figures 8 and 9).
Repeated measure analyses: LS mean estimation for EORTC QLQ-C30 GHS/QoL and functional measures.
Repeated measure analyses: LS mean estimation for EORTC QLQ-C30 GHS/QoL and functional measures.
Repeated measure analyses: LS mean estimation for EORTC QLQ-C30 symptom and individual item measures.
Repeated measure analyses: LS mean estimation for EORTC QLQ-C30 symptom and individual item measures.
Discussion
The superior efficacy of blinatumomab relative to chemotherapy in treating patients with R/R Ph− BCP-ALL observed in the phase 3 TOWER study, as demonstrated by improved overall survival,5 was reflected in the patient-reported HRQL measures. Differences in HRQL favoring blinatumomab vs chemotherapy were observable as early as 8 days after treatment initiation. HRQL changes from baseline, as assessed with the EORTC QLQ-C30 instrument previously validated in other types of cancer,22 were neutral or positive in the blinatumomab group vs neutral or negative in the chemotherapy group. Adverse events may have contributed to the benefit of blinatumomab on HRQL measures as the 2 most common adverse events of grade 3 or higher in both groups, neutropenia and infection, occurred less often in patients treated with blinatumomab vs chemotherapy.5 The decrement in HRQL was especially pronounced for chemotherapy nonresponders. Overall, the magnitudes of changes in HRQL from baseline associated with chemotherapy greatly exceeded those of blinatumomab.
In addition to smaller overall changes in HRQL, blinatumomab was associated with a relative delay in time to clinically meaningful deterioration (≥10-point change) in HRQL measures, with or without the event of death, relative to chemotherapy, with HRs of <1.0 for all EORTC QLQ-C30 scales and P < .05 for 12 of the 15 EORTC QLQ-C30 scales. Taken together with the improved survival of patients treated with blinatumomab, the corresponding delay in HRQL deterioration and the smaller overall magnitude of HRQL change underscore the benefit of blinatumomab therapy in patients with R/R Ph− BCP-ALL, especially when their advanced disease is considered.
In the INO-VATE phase 3 trial, which also used the EORTC QLQ-C30 assessment tool, patients with R/R B-cell ALL treated with inotuzumab ozogamicin reported generally better HRQL as assessed using a mixed-effects model than those who received chemotherapy, with a minimally important difference (MID) of ≥5 and P < .05 reached on 4 measures (physical, role, social function, and appetite).9 In the TOWER MMRM analyses using cycle 1 data, an MID of ≥5 and P < .05 were reached for treatment favoring blinatumomab vs chemotherapy for all HRQL measures except financial difficulties. These analyses from the TOWER and INO-VATE studies support the link between superior clinical efficacy of antibody-based therapy vs conventional chemotherapy and better HRQL in patients with difficult-to-treat R/R ALL.
Limitations
(1) Due to the subjective nature of PRO end points, the open-label design of the TOWER study may have influenced results because patients were aware of their treatment allocation. However, the added burden of a blinded study with alternative routes of administration combined with the stress of not knowing the active treatment could also impact HRQL outcomes and be too burdensome for the patients, especially when survival time for many is extremely limited.23 Given that the mean pretreatment baseline EORTC QLQ-C30 scores were balanced between the groups with comparable completion rates, the impact of the open-label design was likely not substantial. (2) Awareness of response status was likely not a strong source of bias because equal percentages of patients in both treatment groups in the EORTC QLQ-C30 analysis set responded to treatment after cycle 1. Thus, the improved HRQL status reported by patients receiving blinatumomab cannot be attributed to the higher response rates observed by patients treated with blinatumomab in the TOWER study. (3) The EORTC QLQ-C30 analysis set required ≥1 postbaseline score and consequently was smaller than the ITT analysis set, which could potentially have introduced bias. This difference was partially due to the number of patients randomized but not treated (chemotherapy group, n = 25; blinatumomab group, n = 4), and consequently excluded from the EORTC QLQ-C30 analysis set. The most common reason for not being treated was patient request (chemotherapy group, n = 22; blinatumomab group, n = 1). Additionally, differences in missingness between groups could potentially be attributed to greater staff access to patients receiving inpatient blinatumomab infusions; and as fewer patients receiving blinatumomab experienced worsening of symptoms, more may have been physically able to complete the assessments. (4) Hospitalization length of stay may have accounted for some of the differences between groups because those treated with blinatumomab could leave at day 8 or 9 whereas those receiving chemotherapy stayed, based on historical data, an average of about 13 days.24 Unfortunately, the TOWER study captured only the length of stay of unplanned hospitalizations associated with adverse events, so no analysis based on hospitalization time was possible. (5) The MMRM analyses were for HRQL only and did not take into account deaths that may have occurred between day 15 and day 29. Given the higher mortality rate in the chemotherapy group (14.7% vs 8.9% in the blinatumomab group), the HRQL scores could conceivably be biased in favor of chemotherapy. (6) To be consistent with the prespecified secondary end point, the ≥10-point responder definition was used. However, the literature suggests that between-group mean differences should be scale specific.20 Further analyses should be conducted to validate the results of this research. (7) Finally, because the Tower study was not designed to formally test HRQL end points and because there was no adjustment in this analysis for multiple comparisons, all results are descriptive. Therefore, those results where P < .05 do not signify formal statistical significance. As the sample size was small due to discontinuation or death and the study not powered for these measures, differences as a result of random error were possible. Given the descriptive nature of these findings, additional studies are needed to confirm our results.
Strengths of the study
As has been shown in a limited number of phase 3 studies,9,25,26 the collection of HRQL data in patients with acute leukemias is feasible and highly informative, providing that appropriate methodology is used. The EORTC QLQ-C30 questionnaire is a commonly used, reliable, and validated tool,11,27,28 with the majority of available patients completing the questionnaire. The demographics and characteristics of the blinatumomab and chemotherapy groups in the EORTC QLQ-C30 analysis were well balanced, reflecting the randomized study design. Importantly, the consistency of the findings across multiple subscales and analyses demonstrates the robustness of the results. Given that patients with ALL may experience very rapid changes in their circumstances, the weekly assessment of HRQL allowed the dynamics of their condition to be captured.
In conclusion, in this phase 3 study, HRQL measures stabilized in patients treated with blinatumomab regardless of response status, whereas HRQL declined rapidly in patients receiving chemotherapy. As seen in the delay in deterioration in HRQL for patients treated with blinatumomab vs chemotherapy, taken together with a superior overall survival, blinatumomab provided clinically meaningful benefits to patients with R/R Ph− BCP-ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Julie Gegner, employee of Amgen, provided drafts and editorial support.
This work was supported by Amgen Inc., Thousand Oaks, CA.
Authorship
Contribution: M.S.T., Z.Z., H.D., A.S., Z.C., and A.C.S. contributed to study conception and design; M.S.T., P.C., H.D., J.M., A.S., and A.C.S. collected patient data; and M.S.T., Z.Z., H.D., A.S., J.F., Q.T., Z.C., and A.C.S. contributed to data analysis and interpretation.
Conflict-of-interest disclosure: M.S.T. has received research grants from Amgen Inc, Roche, and Regeneron; consulting fees from Amgen Inc, MacroGenetics, and Regeneron; participated in a speakers’ bureau for Amgen Inc; and received other (travel) from Celgene and Amgen Inc. Z.Z., J.F., Q.T., and Z.C. are employed by and own stock in Amgen Inc. H.D. has received research grants from Pfizer, Incyte, Jazz Pharmaceuticals, Kite Pharmaceuticals, and Amgen Inc; honoraria from Pfizer, Incyte, Novartis, Jazz Pharmaceuticals, Agios, Sunesis, Daiichi Sankyo, Karyopharm Therapeutics, Kite Pharmaceuticals, Menarini, Astellas, Janssen, Servier, Seattle Genetics, Cellectis, Celgene, and Amgen Inc; had an advisory role at Pfizer, Incyte, Novartis, Jazz Pharmaceuticals, Agios, Sunesis, Daiichi Sankyo, Karyopharm Therapeutics, Kite Pharmaceuticals, Menarini, Astellas, Servier, Seattle Genetics, Cellectis, Celgene, and Amgen Inc; participated in speakers’ bureaus for Pfizer, Celgene, Incyte, and Amgen Inc, and received travel/accommodations from Pfizer, Incyte, and Amgen Inc; and consultancy for Celgene, Jazz Pharmaceuticals, Amgen Inc, and Chugai/Roche. J.M. has received research grants and consulting fees from Merck Sharp & Dohme (MSD), Pfizer, Gilead, and Basilea; and served on advisory boards for MSD, Pfizer, Gilead, and Basilea. A.S. served as a consultant and on a speakers’ bureau for Amgen Inc. A.C.S. received consulting fees from Celgene, Lundbeck, Shire, Novartis, and Amgen Inc. P.C. declares no competing financial interests.
Correspondence: Andre C. Schuh, Princess Margaret Cancer Centre, 610 University Ave, OPG 6-425, Toronto, ON M4N 1A7, Canada; e-mail: andre.schuh@uhn.ca.