Abstract
T-cell chronic active Epstein-Barr virus (CAEBV) is a rare disease in which EBV is present predominantly in T cells that infiltrate the tissues; patients have high levels of EBV in the blood. If untreated, patients often develop liver failure, hemophagocytic lymphohistiocytosis, coronary artery aneurysms, EBV infiltrating T cells impairing organ function, or T-cell lymphomas refractory to treatment. At present, hematopoietic stem-cell transplantation is the only curative therapy, and it is critical to make a proper diagnosis and initiate transplantation before the disease progresses to an irreversible stage. Specific medications such as high-dose systemic corticosteroids or ganciclovir combined with either histone deacetylase inhibitors or bortezomib may temporarily reduce systemic toxicity associated with T-cell CAEBV and allow the patient time to receive a transplant. Relapses of the disease after transplantation have also occurred, and the use of donor-derived virus-specific T cells may help to treat these relapses.
Introduction
Approximately 95% of adults are infected with Epstein-Barr virus (EBV). In nearly all persons, the virus is latent in B cells. Some immune-compromised persons have virus in B cells as well as T cells.1,2 In other patients, especially those in Asia and South and Central America, EBV is predominantly in T cells, with high levels of EBV in the blood and infiltration of tissues with virus-infected T cells.3,4 The disease, which has been reported less often in the United States,5 is referred to as T-cell chronic active EBV (CAEBV). Patients often present with fever, liver function abnormalities, hepatosplenomegaly, lymphadenopathy, and cytopenias.6 Numerous life-threatening complications can occur, including hemophagocytic lymphohistiocytosis (HLH), coronary artery aneurysms, liver failure, and T-cell lymphomas. Although T-cell CAEBV has more often been reported in children and adolescents,6 the disease has more recently been reported in adults.7 The disease in adults seems to be more aggressive and progresses more rapidly than in children. The disease may have an insidious or acute fulminant course and is difficult to treat once organ dysfunction, especially hepatic insufficiency, occurs. Some patients have high levels of EBV in T cells in the blood and disease localized to the skin in the absence of systemic symptoms, termed EBV hydroa vacciniforme, and the course may be indolent.
Case reports
Case 1
A 25-year-old Latin American woman during her third pregnancy developed jaundice, fevers, and chills. Liver enzyme abnormalities persisted, and she was hypoalbuminemic, with coagulopathy and platelets in the 90 × 109/L range, and had elevated bilirubin. She underwent a liver biopsy, which showed mild focal hepatitis with no obvious evidence of infection. Magnetic resonance imaging of the abdomen revealed mild hepatomegaly, and the spleen was normal in size. The patient was followed as an outpatient, and liver enzymes improved. She became pregnant again 1 year later. Again, she developed elevation of her transaminases while she was pregnant. An ultrasound of the abdomen did not show any abnormalities, and the spleen was not enlarged. She underwent another liver biopsy, which revealed focal lobular sinusoidal lymphocytes. The patient miscarried at 20 weeks. She continued to be followed as an outpatient and was doing well until 3 years later, when she developed recurrent chills and fevers of 102°F. The patient underwent a third liver biopsy, which revealed mixed macro- and microvesicular steatosis. EBV RNA was detected in the liver. Review of the 3 liver biopsies taken over the 4-year period indicated a diagnosis of T-cell CAEBV infection. The patient was treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and acyclovir. However, she progressed, with markedly elevated EBV DNA levels of >200 000 copies/μg DNA, fever to 104°F, abnormal liver function tests (LFTs; bilirubin 13 mg/dL), and worsening disease, with a bone marrow biopsy confirming hemophagocytosis. She was treated with a schedule comprising bortezomib and ganciclovir and had a dramatic response to this therapy, with her EBV DNA level falling to <100 copies/μg DNA. Clinically, she was afebrile and had no adenopathy, and her LFTs and complete blood count were normal.
The patient’s diagnosis was T-cell CAEBV. Her disease seemed to wax and wane, with initially a relatively indolent course, which worsened with the immune suppression of pregnancy. She subsequently received an allogeneic hematopoietic cell transplant (HCT) from an EBV+ 8/10 HLA-mismatched unrelated donor using a reduced-intensity regimen (alemtuzumab administered distally from day −14, fludarabine 150 mg/m2, and melphalan 140 mg/m2), but engraftment failed, with a rapid rise in her EBV DNA levels. A second transplantation was attempted, but the patient died before engraftment of bleeding complications.
Although this patient initially presented with a subacute case of T-cell CAEBV, after a 4-year period, she subsequently presented with signs and symptoms typical of acute presentation. This case therefore illustrates the difficulty the clinician faces regarding timing of allogeneic HCT without the benefit of the retrospectoscope.
Case 2
A 12-year-old white boy presented with a recurrent pustular rash on his face and dorsum of his hands. The rash was associated with sun exposure. It would begin as erythematous papules that rapidly became pustular and then healed with scarring. A biopsy of a lesion showed infiltration of T cells, some with atypia. In situ hybridization for EBV RNA showed that the T cells expressed EBV-encoded RNA (EBER). Further workup showed a normal complete blood count and differential, normal chemistries and LFTs, and normal levels of immunoglobulins and CD4, CD8, CD20, and natural killer (NK) cells. There was no evidence of a T-cell clone in the blood. The EBV viral capsid antigen (VCA) immunoglobulin M was negative, and the EBV VCA immunoglobulin G was positive, indicative of prior infection. The EBV DNA polymerase chain reaction in the blood was 580 000 copies/mL. Sorting of lymphocytes in the peripheral blood showed that EBV was predominantly in T cells. The patient had no history of severe prior infections. There was no family history of a similar rash, lymphoma, or other cancers. The patient has been followed for 6 years, and other than the rash induced by sun exposure, he has had no other problems. His EBV level remains in the range of 400 000 to 600 000 copies/mL.
The patient’s diagnosis was EBV hydroa vacciniforme. This patient’s disease shares many features of T-cell CAEBV, with high levels of EBV in the blood in T cells and EBV RNA in T cells in the skin. Many patients with EBV hydroa vacciniforme who do not have associated systemic symptoms, such as fever, night sweats, weight loss, or hepatitis, may have long periods of good health, with only episodic skin involvement, and may not develop organ dysfunction or T-cell lymphoma.8
Biology of T-cell CAEBV
EBV is known to enter B cells by attachment of the virus to CD21 on the surface of the cells; however, most studies have shown that CD21 is not present on T cells or present at low levels. CD21 is absent from the surface of EBV+ T cells1 ; however, it is possible that CD21 may have been present on the cells and then downregulated after EBV infection. It is uncertain how the virus enters T cells. EBV type 2 can infect T cells in vitro9 ; however, most infections with EBV outside of equatorial Africa and New Guinea are of EBV type 1. In addition to T-cell CAEBV, EBV is present in T cells of healthy Kenyan children infected with EBV type 2,10 in a small percentage of tonsils,11 and in T cells from some patients with T-cell lymphoma or HLH.
Patients with T-cell CAEBV often have an EBV+ T-cell clone in the blood, although oligoclonal and polyclonal EBV+ T cells have also been detected.12 The EBV-infected T cells can be CD4+ or CD8+. The T cells often express a limited set of EBV latency genes, EBV nuclear antigen 1 (EBNA1), and latent membrane proteins 1 and 2. Although these viral proteins are recognized by cytotoxic T cells in healthy persons, patients with CAEBV often have reduced numbers of EBV-specific CD8 T cells13 and impaired EBV-specific cytotoxic T-cell activity.14,15
Serum or plasma cytokines are often elevated in the blood, including interleukin-1β (IL-1β), IL-6, IL-10, IL-13, tumor necrosis factor α, and interferon-γ (IFN-γ).5,12 The elevated levels of some of these cytokines, particularly IFN-γ, tumor necrosis factor α, and IL-6, may contribute to development of HLH. In a large series of patients, HLH was frequently associated with EBV in CD8 T cells.3
Genetics of T-cell CAEBV
T-cell CAEBV is much more common in persons of Asian and South or Central American ancestry than in whites. The reason for this is unclear, but it is likely a result of genetic predisposition. HLA-A*26 is more common in persons from Japan and Taiwan than the United States, and this HLA allele was associated with an increased frequency of CAEBV in Japan and Taiwan.16 Thus far, germ line mutations have not been identified in T-cell CAEBV, although somatic mutations in the EBV-infected T cells may be present. Recent studies have shown that mutations in DDX3X have been detected in T/NK cells from patients with CAEBV.17 DDX3X encodes an RNA helicase that is important for unwinding of RNA and has roles in transcriptional regulation, cell signaling, and viral replication. Mutations in DDX3X have been associated with somatic mutations in numerous malignancies, including T-cell acute lymphoblastic leukemia, chronic lymphocytic leukemia, NK/T-cell lymphoma, and medulloblastoma.18
Diagnosis of T-cell CAEBV
We define T-cell CAEBV as a chronic disease (lasting for at least 6 months), with high levels of EBV in the peripheral blood, in which the virus is predominantly in T cells, and infiltration of tissues with EBV+ T cells in persons who have no known immunodeficiency. Although the standard definition has been a disease lasting for 6 months, some patients may present with a more acute course, perhaps with symptoms that were not fully appreciated, and decisions regarding therapy cannot wait for months. More recent studies have used a definition of disease of at least 3 months in duration.19
Patients with T-cell CAEBV have high levels of virus in the blood. The virus is present predominantly in T cells. In a study of 108 patients with T- and NK-cell lymphoproliferative disease (80 of whom had CAEBV), although the quantity of EBV DNA measured in plasma or in peripheral blood mononuclear cells (PBMCs) showed a significant correlation, the levels of EBV DNA in plasma were ∼10-fold lower in PBMCs in patients with CAEBV, and EBV DNA was not detected in the plasma in 15 (14%) of 108 patients.3 Therefore, we favor quantitation of EBV in whole blood or PBMCs rather than in plasma. Most EBV DNA in the plasma from persons with CAEBV is nuclease sensitive, indicating it is not in virions but likely released from dead cells.12 Prior studies of CAEBV used high levels of antibodies to EBV VCA or early antigen as part of the diagnostic criteria20 ; at that time, the tests were performed by immunofluorescent assay of diluted sera. The titers of EBV-specific antibodies vary widely in controls21 ; therefore, the titer of antibody is not specific for diagnosis of chronic active infection. In addition, EBV-specific antibody assessment is now performed using enzyme-linked immunosorbent assay, which is less useful for quantification at high titers. Thus, quantification of EBV DNA polymerase chain reaction in blood has replaced EBV antibody titers in the diagnosis of CAEBV.
The differential diagnosis of T-cell CAEBV includes NK-cell and B-cell CAEBV as well as other EBV lymphoproliferative diseases, which occur after transplantation or in patients with HIV. T-cell CAEBV can be distinguished from NK- or B-cell CAEBV either by identifying the cell type infected with EBV in tissue or by sorting the peripheral blood and quantifying the number of EBV DNA copies in T, B, and NK cells. It is important to rule out known causes of acquired or congenital immunodeficiency. NK-cell CAEBV has a somewhat better prognosis than T-cell disease,22 although both diseases usually require transplantation for cure (as described in “HCT”). Patients with hydroa vacciniforme or HLH can have EBV in T cells, but the former often have disease limited to the skin; the latter have acute disease, and if they respond to immune suppression and do not have a genetic disorder or central nervous system disease, they often have a good long-term prognosis.
Approach to therapy
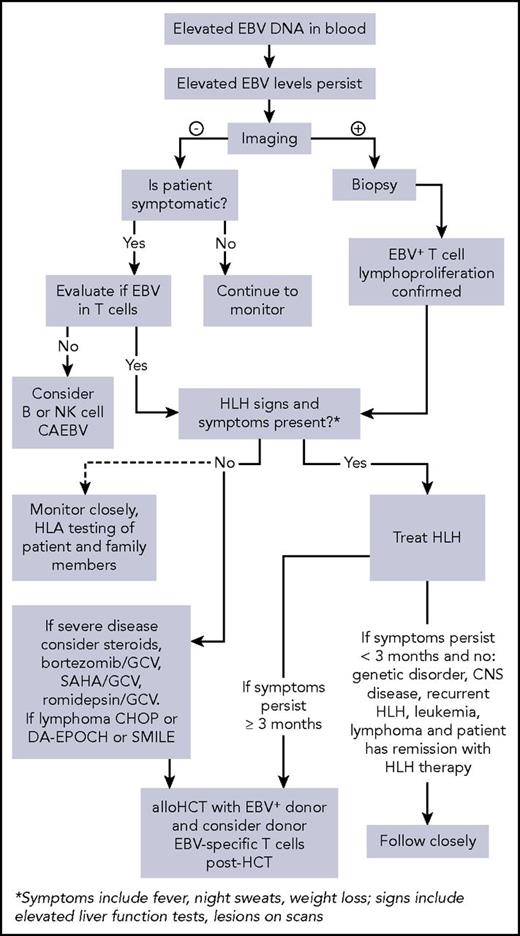
T-cell CAEBV is 1 of the representative EBV-associated T/NK-cell lymphoproliferative disorders, according to current diagnostic criteria.19 Conventional therapies in the past for T-cell CAEBV have included antiviral drugs and/or immune-modulatory agents such as IFN-γ, IL-2, corticosteroids, or cyclosporine A. All these strategies have been used without clear benefit, and currently, a standard treatment approach has not been established. We provide an algorithm that we have used for our approach to patients with T-cell CAEBV (Figure 1).
Algorithm for evaluation and treatment of chronic active EBV. allo, allogeneic; CNS, central nervous system; DA-EPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; GCV, ganciclovir; SAHA, suberoylanilide hydroxamic acid; SMILE, dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide.
Algorithm for evaluation and treatment of chronic active EBV. allo, allogeneic; CNS, central nervous system; DA-EPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; GCV, ganciclovir; SAHA, suberoylanilide hydroxamic acid; SMILE, dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide.
Immunomodulatory therapy
Multiple cases of patients treated with immunosuppressive therapy, including corticosteroids, cyclosporine, or azathioprine, have demonstrated that such therapy may transiently relieve symptoms but does not provide a lasting effect in any patient.3,5 The Japanese group use immunomodulatory therapy as step 1 of a 3-step approach to the treatment of T-cell CAEBV. When a patient is diagnosed with CAEBV, they immediately initiate step 1, which is prednisolone, cyclosporine A, and etoposide,23 but this treatment regimen is then immediately followed by step 2 (chemotherapy) and allogeneic HCT (step 3).
Pulsed high-dose methylprednisolone (2 mg/kg per day) or prednisone (up to 1.5 mg/kg per day) may be required for severe cases of T-cell CAEBV. For patients with HLH symptoms and signs, HLH should be treated with HLH-directed therapy including dexamethasone and etoposide.24,25 Alternatively, if the patient is currently asymptomatic (ie, in remission) or HLH signs and symptoms (eg, fever, night sweats, weight loss, abnormal LFTs, abnormal ferritin levels) are absent and the patient seems too well to proceed to chemoimmunotherapy, careful monitoring and observation can be considered until the condition worsens. However, there is some disagreement among experts in this regard, because a vast majority of patients inevitably do require intensive treatment.
Antiviral therapy
Antiviral therapy with acyclovir inhibits the viral DNA polymerase but not the host-cell polymerase. Therefore, although acyclovir inhibits lytic virus replication, which occurs in epithelial cells, it has virtually no effect on the virus when lymphocytes divide and use the host-cell polymerase to replicate viral DNA. In theory, antiviral therapy might have an effect early in primary infection, when the virus replicates in cells and spreads to other cells. However, once EBV infection is established, acyclovir has no effect on virus-infected T cells in T-cell CAEBV.
Targeting the EBV-infected T cells
For symptomatic patients (without HLH), we hypothesize that 1 potentially effective strategy includes combining ganciclovir, an antiviral agent, with drugs that can induce lytic gene expression in EBV-infected T cells (Figure 2). The latently infected EBV+ T cells are resistant to nucleoside-type antiviral agents because the drugs are not activated in these cells as the EBV-protein kinase (BGLF4) is not expressed.26 Histone deacetylase inhibitors such as vorinostat or romidepsin, proteasome inhibitors such as bortezomib, and short-chain fatty acids such as butyrate induce EBV protein kinase expression in latently infected cells, which phosphorylates ganciclovir, resulting in cell death. One example of such a strategy is using a schedule of bortezomib (velcade) and ganciclovir (or oral valganciclovir) as per a previous clinical trial targeting EBV+ lymphomas (registered at www.clinicaltrials.gov as #NCT00093704). Although there has been anecdotal success in our experience, there are as yet no comprehensive reports documenting the response rate and efficacy of this approach. Typically, patients receive bortezomib (1.3 mg/m2) IV over 3 to 5 seconds on days 1, 4, 8, and 11 and receive ganciclovir IV twice daily on days 1 to 14. We also propose that it is important to use ganciclovir and not acyclovir in this setting. Phosphorylated ganciclovir is toxic and kills infected cells, whereas phosphorylated acyclovir does not kill infected cells (although it does inhibit virus replication). Although oral valganciclovir can be used instead of ganciclovir, we recommend starting with ganciclovir first and then, when the viral load is more controlled, switching to the oral formulation.
Treatment of EBV-infected lymphocytes with bortezomib or histone deacetylase (HDAC) inhibitors along with GCV can induce apoptosis of infected cells. EBV-infected T cells contain episomal EBV DNA (A). Bortezomib and HDAC inhibitors induce virus replication and expression of the EBV PK (B). GCV is phosphorylated by the viral PK, resulting in inhibition of EBV replication; phosphorylated GCV induces cell-cycle arrest and apoptosis of virus-infected cells (C). P, phosphate; PPP, triphosphate.
Treatment of EBV-infected lymphocytes with bortezomib or histone deacetylase (HDAC) inhibitors along with GCV can induce apoptosis of infected cells. EBV-infected T cells contain episomal EBV DNA (A). Bortezomib and HDAC inhibitors induce virus replication and expression of the EBV PK (B). GCV is phosphorylated by the viral PK, resulting in inhibition of EBV replication; phosphorylated GCV induces cell-cycle arrest and apoptosis of virus-infected cells (C). P, phosphate; PPP, triphosphate.
Treatment should be repeated every 21 days for a maximum of 3 courses. We recommend the following for hepatic impairment: for mild impairment (bilirubin ≤1 times upper limit of normal [ULN] and aspartate aminotransferase > UNL or bilirubin >1-1.5 times ULN), no initial dose adjustment required; for moderate (bilirubin >1.5-3 times ULN) or severe impairment (bilirubin >3 times ULN), reduce initial dose to 0.7 mg/m2 in the first cycle; based on patient tolerance, dose escalation to 1 mg/m2 or further dose reduction to 0.5 mg/m2 in subsequent cycles may be considered.
Similarly, arginine butyrate in combination with ganciclovir was administered to 15 patients with refractory EBV+ lymphoid malignancies of B-cell or NK/T-cell origin. Ganciclovir was administered twice daily at standard doses, and arginine butyrate was administered by continuous infusion in an intrapatient dose escalation, from 500 mg/kg per day to 2000 mg/kg per day, as tolerated, for a 21-day cycle. Ten of 15 patients showed significant antitumor responses, with 4 complete responders and 6 partial responder (PRs) within 1 treatment cycle. Among the complete responders, 1 patient had extranodal NK/T-cell lymphoma, and 1 patient had peripheral T-cell lymphoma. Among the partial responders, 1 patient had extranodal NK/T-cell lymphoma, and 1 patient had a subcutaneous panniculitis-like T-cell lymphoma. The combination of arginine butyrate and ganciclovir was felt to be reasonably well tolerated, with biologic activity in vivo.27 However, at present, the drug is not available for clinical use.
Therefore, another approach would to use the histone deacetylase inhibitor romidepsin, which is a potent inducer of EBV replication and in combination with ganciclovir has been shown to kill virus-infected cells in vitro and in a mouse model.28 Romidepsin also markedly induced EBV replication in patients and resulted in elevated liver enzymes and fever in NK/T-cell lymphoma.29 The drug is approved for treatment of cutaneous T-cell lymphoma. Hence, when administered with ganciclovir, romidepsin might be used as an alternative to bortezomib for T-cell CAEBV, although close clinical monitoring would be critical.
Other immunomodulatory/T-cell–directed strategies tried have included IFN-α, hydroxyurea, and lenalidomide, none of which have shown robust success.30 HSP90 inhibitors have also been shown to kill EBV-infected B cells and T cells by reducing the levels of EBV EBNA1 and LMP1. In 1 study, treatment of cells with the HSP90 ganetespib also reduced the level of phosphorylated Akt, delayed the onset of EBV+ B-cell lymphoma, and prolonged survival in SCID mice.31 Treatment of a patient with T-cell CAEBV with ganetespib reduced the percentage of EBV+ T cells in the peripheral blood.32
Chemotherapy
The timing of chemotherapy is a source of debate. Chemotherapy can significantly reduce disease activity as well as the burden of residual EBV-infected T cells. Although it is preferable to substantially reduce viral loads (ideally to 0) before HCT, in practice this is usually not possible. A major goal of chemotherapy is to control visceral disease to reduce the risk of complications associated with HCT. Chemotherapeutic regimens used have included DA-EPOCH and CHOP, which have successfully been used to treat patients with T-cell lymphomas.33,34 In addition, SMILE, a promising chemotherapy for NK-cell malignancies, has also shown some efficacy in a subset of patients with T-cell CAEBV, although the numbers are still small.23,35
For patients with HLH signs and symptoms, initiating HLH-directed treatment is required. Recent data suggest that the HLH-94 protocol, which includes an 8-week induction therapy with dexamethasone, etoposide, and intrathecal methotrexate (when central nervous system disease is present), is preferred to the HLH-2004 protocol, which moved cyclosporine dosing to the beginning of induction and added hydrocortisone to intrathecal therapy. The principal goal is to suppress the lifethreatening inflammatory process that underlies HLH and transition to continuation therapy as a bridge to allogeneic bone marrow transplantation.24,25
EBV-specific T-cell therapies
Studies have shown that autologous EBV-specific T cells directed toward LMP1 and LMP2 induce clinical responses in patients with EBV+ Hodgkin lymphoma and NK/T-cell non-Hodgkin lymphoma without significant toxicity.36,37 However, data suggest that using autologous T cells specific for EBV antigens is not an effective approach for T-cell CAEBV, with all patients ultimately progressing after autologous T-cell therapy.37 For many patients with relapsed or refractory disease and especially patients with CAEBV, allogeneic HCT currently offers the only curative approach, as discussed in “HCT.” Hence, the feasibility of infusing donor-derived LMP-specific T cells posttransplantation into patients with T-cell CAEBV has also been evaluated.38 These preliminary findings support the opinion that donor-derived LMP-specific T cells should be administered early posttransplantation when patients are in remission.
HCT
Retrospective reviews from Japan and the United States of the natural history of CAEBV suggest that most patients die within 10 to 15 years of diagnosis in the absence of HCT.5,7,39,40 Death usually results from organ failure (especially hepatic failure), hemophagocytic syndrome, or lymphoma.
The largest published series of HCT for T-cell CAEBV have come from Japan. In a recent review, 63 patients with controlled CAEBV after chemotherapy underwent a so-called planned HCT.23 The 3-year event-free survival and 3-year overall survival (OS; ± standard deviation [SD]) were both 87.3% ± 4.2% in this setting. Initially, in the late 1990s, HCT conditioning for this disease was fully myeloablative, but more recently, reduced-intensity conditioning (RIC) regimens have been used. However, the optimal conditioning regimen has still not been identified for all patients. The Japanese group asserts that an RIC approach is appropriate when preceded by the successful use of multidrug chemotherapy to obtain disease control. In the same series using the planned HCT approach, patients (n = 54) receiving an RIC regimen using fludarabine, melphalan, ATG, prednisone, and etoposide achieved a 3-year OS (±SD) of 90.7% ± 4.0% vs 66.7% ± 15.7% in patients (n = 9) receiving a fully myeloablative regimen (ie, total-body irradiation [12 Gy] and cyclophosphamide 120 mg/kg and etoposide 900 mg/m2; P < .05).
Again, in this planned setting, no significant difference was observed between RIC plus cord blood transplantation (n = 27; 3-year OS [± SD], 92.6% ± 5.0%), RIC plus HCT (n = 21; 3-year OS [± SD], 90.5% ± 6.4%), and RIC plus peripheral blood stem-cell transplantation (n = 6; 3-year OS [± SD], 83.3% ± 15.2%; P = .74). Engraftment failure was said to be improved when additional melphalan 70 mg/m2 was added to the RIC regimen on day 8, with the complete engraftment rate improving from 6 (66.7%) of 9 to 11 (100%) of 11 (P = .04). More recently, the Blood and Marrow Transplant Clinical Trials Network completed the RICHI (Reduced Intensity Conditioning for Hemophagocytic Syndromes or Selected Primary Immune Deficiencies) trial, which included T-cell CAEBV (protocol 1204). In this trial, patients received alemtuzumab 0.2 mg/kg on days −14, −13, −12, −11, and −10, fludarabine 30 mg/m2 on days −8, −7, −6, −5, and −4, melphalan 140 mg/m2 on day −3 with cyclosporine, and prednisone for graft-versus-host disease prophylaxis. The rationale was that alemtuzumab may have transient effects on CD52+ T-cell CAEBV cells, and additionally, alemtuzumab suppresses the macrophages responsible for hemophagocytic syndromes.
In 2011, we published our experience with HCT for patients with T-cell CAEBV during the last 28 years in the United States.5 Three patients with T- or T/NK-cell CAEBV received HCT for persistent disease followed by EBV/LMP-specific T cells. Two patients received fully ablative regimens with alemtuzumab (busulfan plus cyclophosphamide based or cyclophosphamide plus total-body irradiation based), and 1 patient received a RIC transplantation with thiotepa, cyclophosphamide, and fludarabine. The patients who received the busulfan plus cyclophosphamide–based regimen died as a result of disease, but the other patients (1 with 5/6-mismatched sibling donor and 1 with 5/6-mismatched unrelated donor) remained alive without disease for >1 year. Currently, we are in the process of collecting retrospective data from >40 patients with T-cell CAEBV treated in the United States over the past 5 years to obtain more recent data.
With increasing experience, there is a growing opinion that HCT should be initiated early in the disease process. However, the actual timing of HCT is still a source of debate among experts. Some experts recommend that patients with T-cell CAEBV who are stable should be monitored closely and have a donor identified but not undergo transplantation until the first sign of clinical or laboratory result deterioration. Other experts believe that the safety of allogeneic HCT has improved so substantially that although the manifestations of CAEBV may temporarily be self-limiting, requiring minimum supportive care or only immune suppression, the later in their course that patients are referred for definitive curative therapy (eg, HCT), the more they are at risk for complications, morbidity, and relapse post-HCT. Furthermore, once the disease process becomes fulminant, the prognosis is drastically reduced, because the patient may no longer be eligible for HCT, and if he or she is able to tolerate HCT, the chance of relapse is substantially increased.
Conclusions
T-cell CAEBV is a rare disorder in which EBV-infected T cells infiltrate tissues, and high levels of EBV are present in circulating T cells. In the absence of HCT, the disease has a poor prognosis, and patients may succumb to liver failure, HLH, or T-cell lymphoma. Although numerous treatments have been used empirically to reduce symptoms, only HCT is curative.
Acknowledgments
The authors thank Ryan Kissinger, Visual and Medical Arts, Research Technologies Branch, National Institute of Allergy and Infectious Diseases, for help preparing the figures.
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (J.I.C.).
Authorship
Contribution: C.M.B. and J.I.C. wrote and edited the article.
Conflict-of-interest disclosure: C.M.B. is on the scientific advisory boards of Cellectis, Neximmune, and Torque and has participated in advisory boards for Roche. J.I.C. declares no competing financial interests.
Correspondence: Catherine M. Bollard, Center for Cancer and Immunology Research, Children’s National Health System, The George Washington University, 111 Michigan Ave, NW, Washington, DC 20010; e-mail: cbollard@cnmc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal