In this issue of Blood, Huang et al have provided evidence that altered lipid metabolism is critical for terminal erythropoiesis. A key role is proposed for the PHOSPHO1 gene product, a phosphocholine phosphatase. PHOSPHO1 knockouts (KOs) showed reduced erythroblast proliferation and enucleation in both mice and human erythroid tissues, apparently through energy depletion mediated via inhibition of oxidative phosphorylation of fatty acids and reduced adenosine triphosphate (ATP) production in late glycolysis. This work emphasizes that altered expression of genes involving lipid metabolism are important during late red cell maturation.1
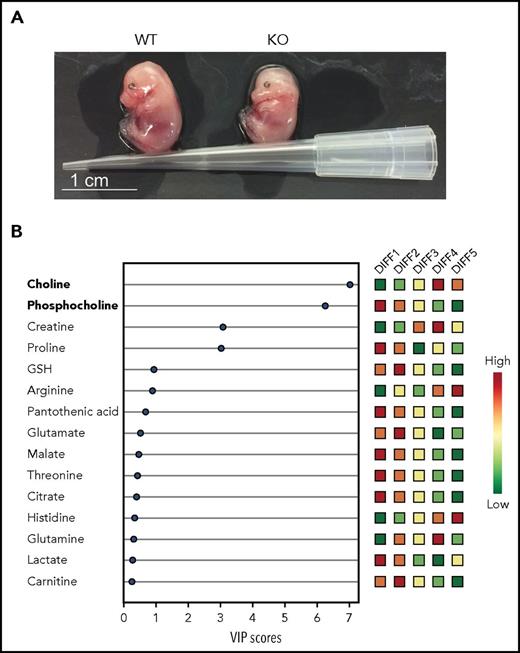
Changes in lipid metabolism are essential for terminal erythropoiesis in mice and humans. (A) Photographs of E14.5 WT and PHOSPHO1 KO mice showing impaired growth and pallor of the mutants, highlighting the importance of phosphocholine metabolism in later red cell maturation. (B) Human CD34+ cells differentiated using a 5-stage in vitro culture, following which lipid metabolites were extracted and analyzed. Relative amounts of polar metabolites (colored boxes, right) with VIP scores (bottom) indicate loss of phosphocholine and increase in choline toward the end of erythroid differentiation. VIP, variable importance in projection; WT, wild-type. See Figures 2B and 6A in the article by Huang et al that begins on page 2955.
Changes in lipid metabolism are essential for terminal erythropoiesis in mice and humans. (A) Photographs of E14.5 WT and PHOSPHO1 KO mice showing impaired growth and pallor of the mutants, highlighting the importance of phosphocholine metabolism in later red cell maturation. (B) Human CD34+ cells differentiated using a 5-stage in vitro culture, following which lipid metabolites were extracted and analyzed. Relative amounts of polar metabolites (colored boxes, right) with VIP scores (bottom) indicate loss of phosphocholine and increase in choline toward the end of erythroid differentiation. VIP, variable importance in projection; WT, wild-type. See Figures 2B and 6A in the article by Huang et al that begins on page 2955.
The mature red cell is unique. Although highly specialized for gas transport, it is much more than an inert receptacle for hemoglobin, with many surprisingly sophisticated properties. Among these, the subtle control of glucose metabolism, cytoskeletal integrity, and membrane permeability by oxygen tension has recently been elucidated.2 During maturation, the developing red cell must both proliferate and undergo considerable modifications to acquire the necessary properties to survive in the circulatory system, where it lacks the ability to synthesize proteins de novo while experiencing profound challenges such as repeated episodes of shear and oxidative stress. Many important changes occur during later erythrogenesis, including loss of the nucleus, shedding of surface markers, establishment of the final surface area/volume ratio, and establishment of a robust but malleable cytoskeleton.3
Our understanding of the processes occurring during erythropoiesis remains partial. Much is known about globin gene switching, which is of particular relevance to a number of the common hemoglobinopathies.4 Some other nonglobin protein changes have also been well studied. These include accumulation of cytoskeletal elements with condensation of spectrin, increased expression of band 3, and acquisition of other requisite membrane transporters.3 Mutations in these proteins are relatively rare, but are sometimes associated with hemolytic anemia and irregularities in red cell shape or volume (such as stomatocytes and spherocytes).5 Elucidation of their molecular causes continues to improve our understanding of red cell physiology.
Diseases involving altered lipid metabolism are arguably less well characterized. As for those involving protein transporters, they can be secondary (ie, subsequent to other diseases). An obvious example here is loss of aminophospholipid asymmetry in a number of hemoglobinopathies such as sickle cell disease. Primary disturbances involving specific gene mutations directly involved in lipid metabolism have been described but are much rarer. Examples include neuroacanthocytosis and phytosterolemia, which result in abnormalities of erythropoiesis and in acanthocytosis and stomatocytic hemolysis, respectively, in addition to their more obvious presenting clinical complications.6,7
Lodish and colleagues have a long track record in cellular and developmental biology, including erythropoiesis. Their current work identifies an additional lipid pathway important for normal red cell development. Subpopulations of developing mouse erythroblasts were separated into 4 terminal stages and were analyzed individually, showing marked variation in lipid content and metabolism. In particular, phosphocholine and its precursor phosphatidylcholine were downregulated during terminal differentiation, whereas sphingomyelin and choline were upregulated and catabolic end products of phosphatidylcholine metabolism became enriched.
By analyzing genes upregulated during terminal differentiation, only 1 pertinent to phosphatidylcholine metabolism was identified, PHOSPHO1. Using short hairpin RNA, Huang et al reduced expression of PHOSPHO1 in mouse fetal erythroid progenitors and found deficiencies in terminal erythroblast differentiation. Cell proliferation and enucleation were reduced, fetal livers showed fewer mature red cells, late embryos were smaller and paler while reticulocytosis was increased (see figure panel A). When early red cells from fetal livers of KO mice were induced to differentiate into erythrocytes in vitro, genes usually upregulated showed reduced expression levels. The number of enucleation events was smaller. Surprisingly, red cell count and volume in postpartum mice were largely normal. However, reticulocytosis suggested that this was probably subsequent to a compensatory stress erythropoiesis. PHOSPHO1 KO mice were also less able to respond to pharmacologically induced stress erythropoiesis using phenylhydrazine. Taken together, these findings suggest that PHOSPHO1 is important for both normal erythropoiesis and response to anemia.
Huang et al went on to investigate the function of PHOSPHO1 in terminal erythropoiesis. In KO mice, accumulation of lipid, reduction in ATP/adenosine 5′-monophosphate ratio, AMPK activation, with reduced levels of oxidative respiration and increased glycolytic flux, were all observed, which indicatives energy depletion, probably through reduction in the supply of lipids for oxidative phosphorylation, together with a greater dependence on anaerobic metabolism. The authors hypothesized that inhibition of phosphatidylcholine and phosphocholine metabolism following PHOSPHO1 KO would lower levels of amino acids glycine and serine, which are essential to normal protein synthesis and are partly supplied through lipid metabolism. Cells would therefore be more dependent on a glycolytic shunt from 3-phosphoglycerate for synthesis of these amino acids. The net effect would be a concurrent reduction in ATP formation from the terminal steps of glycolysis (via pyruvate kinase). Elevated expression of the genes involved in this glycolytic shunt, encoding phosphoserine phosphatase and serine hydroxymethyltransferase, was consistent with their postulate, while glycine or serine supplementation to KO cells, to support/protect glycolysis, largely corrected the falls in enucleation and proliferation.
In the final series of experiments, Huang et al examined the behavior of human CD 34+ stem/progenitor cells induced to proliferate and differentiate into enucleated erythrocytes. The fall in phosphocholine and rise in choline during terminal differentiation paralleled the situation in mice cells (see figure panel B). PHOSPHO1 gene expression was concurrently increased, whereas knockdown of PHOSPHO1 caused marked reduction in cell proliferation and enucleation. Finally, glycine or serine supplementation corrected proliferation rate. These findings suggest a conserved function of PHOSPHO1 in terminal erythropoiesis from mice to humans.
The work of Huang et al serves to emphasize that studies of red cell metabolism during erythropoiesis should not be restricted to that of proteins. It suggests a potential new cause of hemolytic anemia in humans. Recent advances in erythroid culture will provide an excellent opportunity to study in detail changes in lipid metabolism and other key events during late erythroid generation.8 Findings will increase our understanding of normal red cell development and may provide new molecular tools for diagnosis, to correct abnormalities, or to facilitate in vitro erythrogenesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal