Key Points
Thrombophilia was not predictive of recurrent catheter-related deep vein thrombosis in children.
Young age at the time of catheter insertion and lack of administration of anticoagulation were predictive of recurrent events.
Abstract
The role of thrombophilia testing in predicting catheter-related deep vein thrombosis (DVT) after an incident (ie, first) catheter-related DVT in children remains unclear. The present study investigated the association between thrombophilia and recurrent catheter-related DVT. Children with thrombophilia testing, performed according to the clinician’s judgment and the family’s preference, and a history of objectively confirmed catheter-related DVT were included in the study. Recurrent catheter-related DVT after placement of a new catheter was the main outcome. Thrombophilia was classified as minor, major, or none. Analysis was conducted using mixed effect logistic regression. A total of 245 patients had 1,365 catheters inserted; 941 of these catheters were placed after the incident catheter-related DVT. Anticoagulants as treatment or prophylaxis were administered in 78.1% of inserted catheters for at least 50% of the time they were in place. Minor thrombophilia was found in 12.7% of patients, whereas major thrombophilia was seen in 8.2% of children. The incidence rate of recurrent events was 0.23/100 catheter-days (95% confidence interval, 0.19-0.28 catheter-days); 34.3% (95% confidence interval, 28.6%-40.0%) of patients requiring a new catheter after their incident thrombotic event had at least 1 recurrent event. The incidence proportion of bleeding complications was 4.6/100 patients receiving anticoagulation. Young age of the patient at the time of catheter insertion and lack of administration of treatment or prophylactic doses of anticoagulant were predictive of recurrent events. In contrast, thrombophilia was not predictive of recurrent catheter-related DVT during subsequent catheter insertions among tested patients. Our findings suggest that thrombophilia testing to predict recurrence in these patients may be unnecessary.
Background
Thrombophilia refers to the presence of traits that are associated with hypercoagulability and increased frequency of thrombotic events.1
The most common risk factor for venous thrombotic events in children is the presence of a central catheter.2-4 The importance of thrombophilia testing in children with catheter-related venous thrombosis remains uncertain.1,5 For example, a recent systematic review investigating the association between thrombophilia and the prevalence of central venous catheter-related thrombosis reported that the presence of some thrombophilia traits, such as high factor VIII levels, protein C deficiency, and factor V Leiden, confer an increased risk for thrombosis. Nonetheless, the authors of the study concluded that thrombophilia testing was not recommended in children with catheter-related thrombosis, in view of the relatively low incidence of positive thrombophilia results, the heterogeneity of studies and definitions of thrombophilia, and the weak association between thrombophilia and the outcome.6
Importantly, the management of pediatric patients after sustaining an initial episode of catheter-related thrombosis is unclear. A recently published survey of pediatric hematology-oncology specialists showed the heterogeneity of opinions in this area.7 Practitioners were asked how they would manage a child with a history of catheter-related deep vein thrombosis (DVT) and requiring subsequent catheter placement. Approximately one-third (37%) answered they would offer anticoagulant prophylaxis, a quarter (24%) said they would offer prophylaxis depending on the results of thrombophilia testing, and more than a third of respondents (39%) answered they would not consider anticoagulant prophylaxis. The range of responses observed may be a result of the low level of evidence (level 2C) recommending anticoagulation prophylaxis to children with a previously diagnosed catheter-related DVT in whom a new catheter is placed, and the absence of recommendations regarding thrombophilia testing in such scenarios.8
The lack of guidance regarding the role of thrombophilia testing for the clinical management of children with incident catheter-related thrombosis underscores the need for further studies in this area to understand whether testing in these patients is warranted. Such studies will be relevant to inform clinical practice and are expected to have an effect on healthcare costs.
The primary objective of the study was to investigate the role of thrombophilia testing in predicting recurrent catheter-related DVT in children with an incident catheter-related event and requiring subsequent catheter insertions. The secondary objective of the study was to explore additional predictors of recurrent catheter-related DVT in pediatrics.
Methods
The present retrospective longitudinal study reviewed charts of children aged 0 to 18 years with an incident event of catheter-related DVT affecting the upper or lower limbs, diagnosed between 1994 and 2014, who required further catheter placements after their incident thrombotic event. The study was conducted at The Hospital for Sick Children (SickKids), and was approved by the Research Ethics Board at SickKids. Informed consent was waived.
The main outcome of the study was recurrent catheter-related DVT associated with subsequent catheter insertion. Recurrent DVT was defined as an imaging-proven new catheter-related thrombosis, either contiguous or noncontiguous (ie, affecting the same extremity as the index DVT or a different extremity). Extension of the incident event to a contiguous venous segment discovered unintentionally on a follow-up ultrasound was not considered a recurrence. Recurrent thrombotic events were classified as symptomatic or asymptomatic. Asymptomatic events included all new events incidentally found by imaging performed to assess vessel patency or for surveillance purposes, without clear indication of signs or symptoms of DVT.
The main predictor of the study was thrombophilia, which was classified as none, minor, or major. Minor thrombophilia included heterozygous factor V Leiden or prothrombin gene mutation and high lipoprotein (a) or high factor VIII levels (ie, levels above the 95th percentile for age-appropriate values). Major thrombophilia consisted of antithrombin, protein S, or protein C deficiency (ie, levels below age-adjusted values), positive lupus anticoagulant or anticardiolipin antibodies, or homozygous factor V Leiden or prothrombin gene mutation. With the exception of genetic tests, abnormal findings were labeled as such only if confirmed on 2 separate occasions, at least 12 weeks apart, and outside the acute phase of DVT.
For inclusion in the study, patients had to have a minimum thrombophilia panel consisting of antithrombin, protein S, protein C, factor V Leiden, and prothrombin gene mutation testing, unless a single abnormality was otherwise identified and confirmed as described earlier. Thrombophilia testing was performed according to previously reported laboratory protocols.9 The panel was requested according to the judgment of the thrombosis physician and the family’s preference.
Additional variables collected were as follows:
Characteristics of the patients: Age at the time of catheter insertion (in months), age at the time of first DVT, sex, underlying condition, and blood group. For analysis, age at the time of catheter insertion was categorized as less than 1 month vs 1 month or more because of the nonnormal distribution of this variable. Blood group was categorized as O vs non-O, and underlying condition was categorized as cardiac, complex, or other. Diseases leading to organ transplant, genetic disorders with complications, and diseases with neurologic complications were classified as complex. Other diseases included cancer, inflammatory and infectious diseases, prematurity, and congenital diaphragmatic hernia.
Characteristics of the catheter: Type (temporary central venous catheter, peripherally inserted central catheter, or totally implantable venous access device [port]), length of catheter permanence, and year of placement. For analysis, length of catheter placement was categorized as 7 or fewer days or more than 7 days, and year of placement as before 2000, between 2000 and 2005, between 2006 and 2010, and after 2010.
Anticoagulant drugs: Information regarding anticoagulants administered during the time the catheter was in place included intensity (none, treatment dose, or prophylactic dose [ie, 50% of treatment dose]), type of drug (unfractionated heparin, low-molecular-weight heparin, warfarin, or combined treatment), and number of days anticoagulants were given per catheter. For analysis, anticoagulation was considered as administered if given for at least 50% of the time the catheter was in place. In addition, we explored the role of anticoagulation given for at least 50% of the approximate median time to recurrent events. Information regarding the frequency and type of bleeding complications during anticoagulation was also collected. Bleeding events were classified as major, clinically relevant nonmajor, and minor, as per existing recommendations.10
Statistical analysis: Descriptive data were summarized as percentages and ratios or using appropriate measures of central tendency and dispersion, as appropriate. The incidence proportion of bleeding events per anticoagulated patient and the incidence rate and proportion of recurrent events were estimated.
χ-squared test and Wilcoxon sum rank test were used to compare the general characteristics of patients with and without recurrent events.
A random intercept random slope mixed effect multivariable logistic regression model was used to study predictors of the outcome. The approach allows accounting for repeated events in the same patient (ie, repeated catheter insertions). Predictors associated with the outcome at the 0.20 α level in univariable analysis were considered for multivariable analysis. The main predictor, thrombophilia, was kept in the model regardless of significance. Collinearity among predictors was investigated. The between-patient variance to total variance ratio (intraclass correlation coefficient, ICC) and the within-patient variance (1-intraclass correlation coefficient) were estimated for the final model.
The following exploratory analyses were carried out:
Predictors of symptomatic recurrence were investigated (ie, assuming that asymptomatic events were nonevents).
Anticoagulation was evaluated as a predictor of recurrent events only when given for at least 50% of the approximate median time to recurrent events; time to recurrent events was based on the number of days from the placement of the new catheter to the diagnosis of the recurrent event.
α level was set at 0.05. Statistical analysis was performed with the package lme411 for R (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
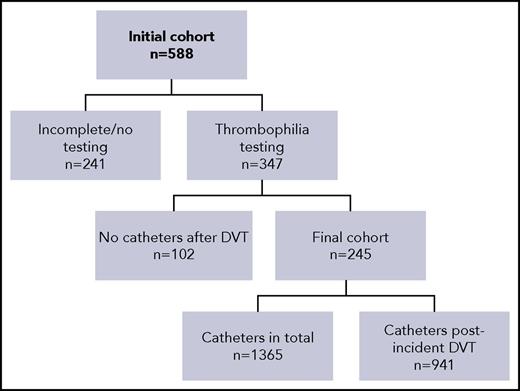
A total of 588 patients diagnosed with an incident catheter-related DVT were identified and screened for inclusion in the study. Two hundred forty-one patients (41.0%) had no or incomplete testing, as defined in Methods, and were not considered in the main analysis of the study. Of the 347 remaining patients, 102 did not need new catheter placement after their incident catheter-related DVT. Therefore, the remaining 245 patients, who did require a subsequent catheter placement after their incident DVT, were considered in the main analysis of the study (Figure 1).
Characteristics of the patients
The characteristics of the 245 patients included in the study are shown in Table 1. The majority of patients were male, the most common underlying condition was cardiac disease (105/245, 42.9%), and the most common blood group was O, Rh+ (86/245, 35.1%). The prevalence of minor thrombophilia among these 245 patients was 12.7% (n = 31), whereas major thrombophilia accounted for 8.2% (n = 20) of abnormal results. The most common thrombophilia findings were high factor FVIII levels (overall frequency, 5%) in the minor thrombophilia group and positive anticardiolipin antibodies or protein S deficiency in the major thrombophilia group (overall frequency of 2% for each abnormality). Of note, the frequency of thrombophilia when considering the entire cohort of 347 tested patients was 14.1% (49/347) for minor thrombophilia and 6.9% (24/347) for major thrombophilia.
Characteristics of the patients
| Variable . | Value . |
|---|---|
| Age at the time of first catheter insertion, (median, 25th-75th percentile), mo | 1.0 (0.1-14.1) |
| Age at the time of first DVT, (median, 25th-75th percentile), mo | 3.6 (0.7-30.5) |
| Age at the time of first postincident DVT catheter insertion, (median, 25th-75th percentile), mo | 6.0 (0.9-34.5) |
| Sex, n (%) | |
| Male | 143 (58.4) |
| Female | 102 (41.6) |
| Underlying condition, n (%) | |
| Cardiac disease | 105 (42.9) |
| Complex* | 27 (11.0) |
| Other | |
| Cancer | 21 (8.6) |
| Inflammatory/infectious | 18 (7.3) |
| Congenital diaphragmatic hernia | 15 (6.1) |
| Prematurity | 13 (5.3) |
| Not classified | 46 (18.8) |
| Thrombophilia findings, n (%) | |
| None | 194 (79.3) |
| Minor | |
| High FVIII levels | 12 (5.0) |
| FVL heterozygous | 11 (4.5) |
| PTG heterozygous | 4 (1.6) |
| Combined† | 2 (0.8) |
| Lipoprotein (a) | 2 (0.8) |
| Major | |
| ACLA | 5 (2.0) |
| Protein S deficiency | 5 (2.0) |
| Combined‡ | 4 (1.6) |
| Protein C deficiency | 3 (1.2) |
| AT deficiency | 2 (0.8) |
| FVL homozygous | 1 (0.4) |
| Blood group, n (%) | |
| O+ | 86 (35.0) |
| O− | 12 (5.0) |
| A+ | 83 (34.0) |
| A− | 6 (2.5) |
| B+ | 43 (17.5) |
| B− | 4 (1.6) |
| AB+ | 9 (3.6) |
| AB− | 2 (0.8) |
| Variable . | Value . |
|---|---|
| Age at the time of first catheter insertion, (median, 25th-75th percentile), mo | 1.0 (0.1-14.1) |
| Age at the time of first DVT, (median, 25th-75th percentile), mo | 3.6 (0.7-30.5) |
| Age at the time of first postincident DVT catheter insertion, (median, 25th-75th percentile), mo | 6.0 (0.9-34.5) |
| Sex, n (%) | |
| Male | 143 (58.4) |
| Female | 102 (41.6) |
| Underlying condition, n (%) | |
| Cardiac disease | 105 (42.9) |
| Complex* | 27 (11.0) |
| Other | |
| Cancer | 21 (8.6) |
| Inflammatory/infectious | 18 (7.3) |
| Congenital diaphragmatic hernia | 15 (6.1) |
| Prematurity | 13 (5.3) |
| Not classified | 46 (18.8) |
| Thrombophilia findings, n (%) | |
| None | 194 (79.3) |
| Minor | |
| High FVIII levels | 12 (5.0) |
| FVL heterozygous | 11 (4.5) |
| PTG heterozygous | 4 (1.6) |
| Combined† | 2 (0.8) |
| Lipoprotein (a) | 2 (0.8) |
| Major | |
| ACLA | 5 (2.0) |
| Protein S deficiency | 5 (2.0) |
| Combined‡ | 4 (1.6) |
| Protein C deficiency | 3 (1.2) |
| AT deficiency | 2 (0.8) |
| FVL homozygous | 1 (0.4) |
| Blood group, n (%) | |
| O+ | 86 (35.0) |
| O− | 12 (5.0) |
| A+ | 83 (34.0) |
| A− | 6 (2.5) |
| B+ | 43 (17.5) |
| B− | 4 (1.6) |
| AB+ | 9 (3.6) |
| AB− | 2 (0.8) |
ACLA, anticardiolipin antibody; AT, antithrombin; FVL, factor V Leiden; PTG, prothrombin gene mutation.
Complex: diseases leading to organ transplant, genetic disorders with complications, diseases with neurologic complications.
High FVIII and heterozygous FVL (n = 2).
Protein C and protein S deficiency (n = 1)/ACLA and heterozygous FVL (n = 1)/AT and protein S deficiency (n = 1)/AT deficiency and heterozygous FVL (n = 1).
The most frequent site for incident thrombosis in the 245 patients was the lower extremity (129/245, 53%). Five percent of the included patients had an initial catheter-related portal vein thrombosis.
Characteristics of the catheters
The 245 patients included in the study had a total of 1,365 catheters, accounting for 67 531 catheter-days. Nine hundred forty-one catheters of the 1365 catheters were placed after the incident thrombotic event, for a total of 45 833 catheter-days. The median number of catheters placed postincident DVT was 5 per patient (25th-75th percentile, 3-9 catheters; range, 2-24 catheters). The median time of permanence of the new catheters was 4 days (25th-75th percentile, 1-23 days; range, 1-2870 days). The most frequent types of new catheter were temporary central venous catheters (697/941, 74.1%), followed by peripherally inserted central catheter (232/941, 24.7%). Ports represented only 1.3% (12/941) of the catheters placed after the incident DVT.
Anticoagulation and bleeding complications
Anticoagulation, as defined in Methods, was used in 67.6% (636/941) of the new catheters at treatment doses. Prophylactic doses only were administered in 10.5% of the new catheters (99/941). The most commonly used anticoagulant was unfractionated heparin (466/636, 73.3%), followed by low-molecular-weight heparin (287/636, 45.1%), and warfarin (20/636, 3.1%). Tissue plasminogen activator was used in 2 catheters. When considering patients as the unit of analysis, 218/245 children (89.0%) received anticoagulation at either treatment or prophylactic doses for at least 50% of the time they had a catheter in place.
Eleven bleeding complications were recorded in 10/218 patients receiving anticoagulation for at least 50% of the time, resulting in an incidence proportion of 4.6 bleeding events per 100 patients receiving anticoagulants. Five of the 11 events were classified as major and included gastrointestinal bleeding (n = 3), pulmonary bleeding (n = 1), and postcardiopulmonary bypass bleeding episode that required blood transfusions (n = 1). Six events were classified as minor and included oral/nasal bleeding (n = 4), soft-tissue hematoma (n = 1), and bleeding at the injection site (n = 1). The incidence proportion of minor and of major bleeding was 2.3 per 100 anticoagulated patients.
Recurrent events
One hundred seven recurrent thrombotic events were documented in 84 children throughout the 45 833 catheter-days of follow-up postincident DVT. This results in an incidence rate of 0.23 events per 100 catheter-days (95% confidence interval [CI], 0.19-0.28), and an incidence proportion of 11.4 events per 100 new catheters (95% CI, 9.5-13.6); 34.3% (95% CI, 28.6%-40.0%) of patients requiring a new catheter insertion after their incident thrombotic event had at least 1 recurrent event. The median number of recurrent thrombotic events was 1 per patient (range, 1-4).
The median time to recurrence from the incident catheter-related thrombotic event was 84 days (25th-75th percentile, 12-335 days). The median time to recurrence from the time the new catheter was inserted was 11 days (25th-75th percentile, 4-44 days).
In terms of the distribution of thrombotic events, only 17 of 107 recurrent events (15.9%) occurred in the same location as the incident DVT, whereas the remaining 90 recurrent thrombotic events (90/107, 84.1%) involved a different limb. Sixty percent of all recurrences were located in the upper extremities (76.5% [13/17] of local recurrences and 56.7% [51/90] of distant recurrences). The remaining recurrences affected the lower extremities.
Sixty-six of the 107 recurrent events (61.7%) were symptomatic. The most common symptoms included limb or facial edema, altered limb perfusion, pain, redness, and catheter dysfunction.
Patients who had a recurrent event did not differ significantly from patients without a recurrent event in terms of sex (58.3% vs 59.0% male patients in each group; P = 1.00), underlying condition (cardiac, 47.6% vs 40.4%; complex, 13.1% vs 9.9%; and other, 39.3% vs 49.7% in each group, respectively; P = .31), blood group (59.5% vs 59.0% of non-O blood group in each group, respectively; P = 1.00), or thrombophilia (no thrombophilia, 75.0% vs 81.4%; minor thrombophilia, 15.5% vs 11.2%; major thrombophilia, 9.5% vs 7.4% in patients with and without recurrence, respectively; P = .52). Only age at the time of first catheter insertion (in the patient’s life) differed significantly between groups (median age, 0.4 months vs 1.6 months in patients with and without recurrence, respectively, P = .005).
Univariable and multivariable analysis
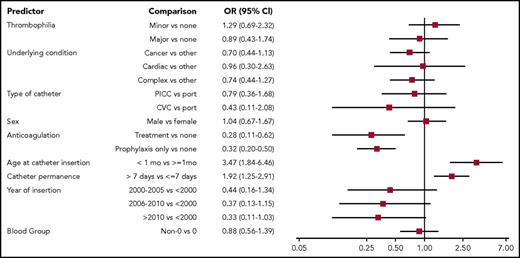
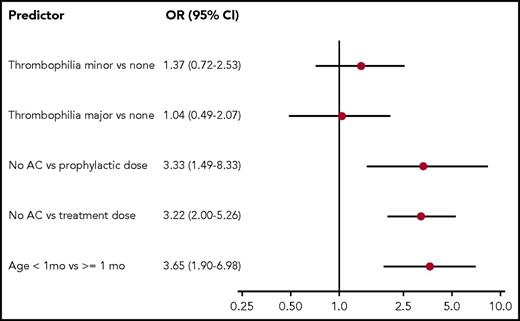
The results of univariable analysis are shown in Figure 2. All the predictors, except sex and blood group, met the criteria to be considered for multivariable analysis. The final model, which is shown in Figure 3, indicates that thrombophilia was not associated with recurrent catheter-related DVT. In contrast, age at least 1 month at the time of postincident DVT catheter insertion and the use of anticoagulation treatment or prophylaxis for at least 50% of the time the new catheter was in place were associated with lower odds of recurrent thrombotic events. There was no association between the remaining variables (underlying condition, type of catheter, catheter permanence, and year of insertion) and the outcome.
Univariable analysis for the outcome recurrent thrombotic event. AC, anticoagulation; CVC, temporary central venous catheter; OR, odds ratio; PICC, peripherally inserted central catheter.
Univariable analysis for the outcome recurrent thrombotic event. AC, anticoagulation; CVC, temporary central venous catheter; OR, odds ratio; PICC, peripherally inserted central catheter.
The intraclass correlation coefficient showed that most of the overall variation in the outcome (94.0%) was explained by within-patient variability rather than by between-patient variability.
Exploratory analyses
The first exploratory analysis showed no change in the final predictors and direction of relationship with the outcome (ie, symptomatic thrombotic recurrences). For the purpose of simplicity in the second analysis, we considered median time to recurrence as 15 days. The model of the second exploratory analysis did not change when considering anticoagulation given for at least 8 of the first 15 days the catheter was in place (ie, or at least 50% of the time of permanence if permanence was less than 15 days) as a predictor variable. Of note, only 6 catheters remained in place for longer than 15 days in patients receiving no anticoagulation at any point beyond the first 15 days; no recurrences were seen in this small sample. In this second model, the odds of recurrent thrombotic events were 2.44 (95% CI, 1.14-5.56) for no anticoagulants vs prophylactic doses of anticoagulants and 3.22 (95% CI, 1.89-7.14) for no anticoagulants vs treatment doses. Thrombophilia remained nonpredictive of the outcome in the 2 exploratory analyses.
Discussion
The present study reports the occurrence of recurrent DVT in 245 children with catheter-related events followed up at SickKids, a tertiary care institution.
The prevalence of thrombophilia traits in the cohort was similar to that reported in children with catheter-related thrombosis in a published meta-analysis,6 except for lower rates of high factor VIII levels (5.0% vs 11.0%), and lipoprotein (a) (0.8% vs 11.0%) found here. The higher frequency of those traits in the meta-analysis may be explained by the inclusion of several studies that did not confirm abnormal findings, as well as the inclusion of a less racially diverse population in some of the studies. The frequency of some thrombophilia traits can be expected to differ based on the racial background of the study population.12-15
When analyzing the time of catheter permanence, we found that 60.0% of patients had a catheter for less than 10 days, which would explain the difference between the median duration of catheter placement (4 days) and the mean duration (49 days). In addition, there was a relatively large number of catheters placed postincident DVT (median, n = 5) in our population. Both observations might be a result of the needs of the patients followed up at our institution, who require numerous catheters for a short time.
In this study, thrombophilia was not associated with an increased risk for catheter-related recurrent thrombotic events. This is contrary to the results published in a meta-analysis of studies looking into the effect of inherited thrombophilia on recurrent thrombotic events in children.16 The authors of the study found that most thrombophilic traits, except for factor V Leiden and high lipoprotein (a), were significantly associated with recurrence. Nonetheless, the studies in the meta-analysis differ widely in several aspects, such as whether traits were confirmed with a second testing, the time of thrombophilia testing with regard to DVT diagnosis, the types of thrombotic events considered (arterial, different locations), and trigger of those events (any trigger, only noncatheter related events, etc.). For example, the second largest study of the meta-analysis, reporting an association between thrombophilia and thrombosis recurrence, only included patients with noncatheter-related thrombosis.17
In addition, the meta-analysis of thrombophilia and catheter-related thrombosis conducted by Neshat-Vahid et al found that some traits (protein C deficiency, high factor VIII, and factor V Leiden) were associated with catheter-related thrombosis.6 However, these findings apply largely to incident events, although whether events were incident or recurrent was not always clarified in the original articles. The same study reported a likely stronger association between thrombophilia and symptomatic thrombosis. Nonetheless, our exploratory analysis showed no difference in the association when asymptomatic events were classified as nonevents. It must be pointed out that identification of symptoms in the pediatric population can be challenging, particularly in younger patients. Symptoms can range in their severity, and milder manifestation, could be easily missed, as they depend on the observer. We have shown the difficulty proxies find in establishing pain in younger patients,18 and that limb edema can be perceived differently by patients as compared with proxies and clinicians.19
In a publication of the Leiden Thrombophilia Study, the authors reported that among adult patients (median age, 45 years), males, females receiving oral contraceptives, patients with idiopathic thrombotic events, and patients with 2 or more thrombophilic traits had a higher risk for recurrent thrombosis. However, individual thrombophilic traits had no major effect on the risk for recurrence, and were deemed by the authors as weak determinants of this outcome.20 A second study in a cohort of unselected adult patients (median age, 67 years) also showed that thrombophilia testing played no role in predicting recurrent events.21 In fact, clinical risk factors remain the most relevant predictors of recurrence among adults.22
Furthermore, we found no association between O and non-O blood groups and recurrent thrombosis. A study conducted in adult patients showed a higher risk for recurrent events among non-O as compared with O blood group patients. However, the study focused on adults with unprovoked DVT who had discontinued anticoagulation.23 A second study enrolling adult patients with an incident pulmonary embolism also reported association between B blood group phenotype and recurrent venous thrombotic events.24 There was no association between specific blood types (A, B, AB, or O) and recurrence in our cohort (data not shown). Importantly, the overall frequency of O group patients and non-O patients in the cohort was similar to that reported in Canada (40.0% and 46.0% O group vs 60.0% and 54.0% of non-O group in the study and Canada, respectively).25
Catheters are the most commonly recognized trigger for thrombosis in pediatrics. The overall prevalence of imaging-proven catheter-related thrombotic events has been estimated at 20.0%,26 which is slightly lower than the frequency of recurrences found here (34.3%). Hence, focusing on preventing recurrent thrombosis in patients who sustain an incident event may be more cost-effective than preventing the incident event.
The present study provides relevant information to this end, as it has identified the use of anticoagulation in either treatment or prophylactic doses as a factor that was associated with decreased odds of DVT recurrence if given for at least 50% of the time the catheter remained in place. Moreover, exploratory analysis suggests that clinicians could even consider administering secondary anticoagulant prophylaxis for a minimum of 8 days within the first 2 weeks after catheter placement (or 50% of the time if permanence is shorter than 15 days). This intervention would decrease the time of exposure to anticoagulation and its inherent risk of bleeding. Furthermore, it would decrease or obviate the need for monitoring levels of anticoagulant drugs. Prophylaxis is not recommended in adult patients with cancer with catheters, 1 of the most studied groups of patients.27 Interestingly, Geerts suggested prophylaxis could have a role in patients with previous thrombosis, although there were virtually no data to support this recommendation.28 Data regarding anticoagulation to prevent recurrent catheter-related events in children are equally scarce, and although anticoagulation has not been found to be protective against a first episode of catheter-related thrombosis in pediatrics,26 it appears to have a role in preventing recurrent events, as suggested by our data. This information can help guide clinical practice, as current guidelines are largely based on expert opinion.8
Importantly, the incidence of bleeding events found here falls within the lower end of the wide range of frequency reported in children receiving unfractionated heparin or low-molecular-weight heparin.29-31
We also found that young age at the time of postincident DVT catheter insertion predicted recurrent events. This might be explained by the higher risk for thrombotic complications in the neonatal period because of mechanical factors and the derangement of a developing hemostatic system observed in sick neonates.
At the same time, our study provides information that could potentially affect healthcare costs, given that thrombophilia testing did not help predict thrombotic recurrences. In fact, positive thrombophilia findings did not predict thrombotic recurrences even when considering symptomatic events only. Moreover, the between- and within-patient variability explained by the model implies that the outcome varied more across occasions in a single patient rather than among patients. This finding also supports the lack of value of thrombophilia testing found here, as it suggests that the specific circumstances surrounding the placement of a new catheter might be the major contributors to the final outcome.
There are limitations to our study. First, its retrospective design requires careful data collection. To increase accuracy, extracted data were double-checked and a third researcher resolved disagreements. Second, the study included a selected cohort of patients, as almost half the initial cohort of patients with an incident catheter-related event was not tested for thrombophilia or testing was incomplete, and it is possible that patients not tested differ from patients who were tested (selection bias). Nonetheless, there was no statistically significant difference in the prevalence of recurrent events between the 241 patients with incomplete or no thrombophilia testing (n = 51, 21.2%) compared with the 347 tested patients (n = 88, 25.4%; P = .33). Moreover, as noted in Methods, testing was performed according to the clinician’s judgment (targeted testing) or the family’s preferences. The panel is routinely offered to all families followed-up in clinic, and each family makes the ultimate decision regarding testing. Third, although it could be thought that inclusion of 2 patients with a single abnormality (ie, targeted testing, patients did not complete all 5 thrombophilia tests) could introduce selection bias, a sensitivity analysis excluding these 2 patients did not change the study results (data not shown). Fourth, the absolute number of patients with major or minor thrombophilia was relatively small, and the study was not formally powered to detect an association between thrombophilia and recurrent catheter-related DVT. However, the observed magnitude of the association between minor and major thrombophilia and the outcome does suggest that these do not meaningfully increase the odds of recurrent thrombotic events. Moreover, the study population represents all the catheter-related events recorded at a large tertiary center. Furthermore, we have accounted for the repeated events in the same patient and adjusted for several potential confounders. Although the analysis of anticoagulant administration as a continuous variable would have been preferable, it was not possible because of nonparametric distribution of data, despite efforts at transformation. Last, anti-β-2 glycoprotein I antibodies were not measured in this cohort of patients, and its role remains to be established in this setting.
In summary, the present work showed the lack of value of thrombophilia testing in predicting recurrent DVT in a cohort of tested children who sustained catheter-related thrombotic events, and highlights the value of anticoagulation and age at the time of postincident DVT catheter placement in predicting recurrence. The potential use of a short course of anticoagulation to prevent recurrent events remains to be investigated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank H. Said, H. Rotz, C. Delayun, and N. Parmar for their contribution to data collection and the Comprehensive Research Experience for Medical Students Program, University of Toronto and Sanofi-Aventis for supporting their participation.
M.L.A. receives support from a Canadian Institutes of Health Research Postdoctoral Fellowship.
Authorship
Contribution: M.L.A. performed outcome assessment, data collection, analyses and interpretation, and wrote the manuscript; N.A., T.T.V., K.B., P.K., and N.Y. collected data and reviewed the manuscript; S.S. performed data analyses and interpretation and reviewed the manuscript; S.W. reviewed the manuscript; and L.R.B. designed the study and wrote the manuscript.
Correspondence: Leonardo R. Brandão, Division of Hematology/Oncology, The Hospital for Sick Children, Toronto, ON M5G-1X8, Canada; e-mail: leonardo.brandao@sickkids.ca.