TO THE EDITOR:
The thrombopoietin receptor agonist eltrombopag (EPAG) has been shown to restore hematopoiesis in patients with severe aplastic anemia (SAA) refractory to immunosuppressive therapy and to significantly improve response rates when used in combination with immunosuppressive therapy in treatment-naïve patients.1-3 Patients with bone marrow failure are frequently iron overloaded as a result of chronic red cell transfusions. Recent in vitro studies suggested that EPAG mobilizes intracellular iron via direct chelation.4,5
To investigate the clinical significance of this observation, we analyzed plasma iron and ferritin levels in patients enrolled in 2 prospective clinical trials that used EPAG monotherapy to treat refractory severe aplastic anemia (refSAA; NCT01891994) or moderate aplastic anemia (MAA; NCT01328587). In patients with refSAA, EPAG was administered over a period of 6 months at a fixed dose of 150 mg once per day. Patients were evaluated for response after 3 and 6 months of treatment at the National Institutes of Health Clinical Center. Some patients discontinued the study before reaching the primary end point at 6 months. Patients with MAA were treated in a dose-escalating fashion (150-300 mg once per day) until the primary response end point of 3 to 4 months. Responding patients on both protocols continued EPAG on an extension arm until they met criteria for a robust hematologic response, at which time the drug was discontinued. In a few patients, EPAG was later restarted because of declining blood cell counts. Iron studies were performed at baseline, at response evaluations, and at periodic follow-up visits (every 6 months while receiving EPAG and at least once per year after EPAG was discontinued in responding patients).
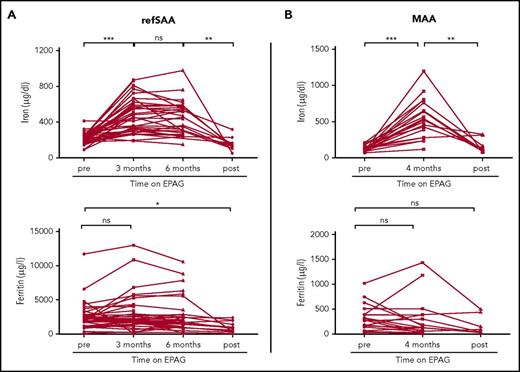
For the refSAA trial, patients with iron studies available from baseline and at least 1 follow-up time point at 3 and/or 6 months of EPAG treatment were included in the analysis (38 of 40 patients enrolled in the trial; supplemental Table 1, available on the Blood Web site). In refSAA patients, iron levels significantly increased from baseline and were measured 3 months after initiation of EPAG treatment (baseline, 213.9 ± 9.6 µg/dL vs after 3 months, 475.7 ± 30.4 µg/dL; P < .001; n = 38; Figure 1A). Of note, 36 of 38 refSAA patients were transfusion-dependent for packed red blood cells at study entry, which explains elevated baseline plasma iron levels. With continuing treatment, plasma iron concentrations remained elevated at the 6-month primary end point (441.6 ± 33.7 µg/dL; n = 30; Figure 1A) but did not further increase compared with the 3-month measurements. In 12 patients, data from studies of plasma iron were available after EPAG was discontinued for nonresponse at the primary 6-month end point (n = 4) or as a result of reaching robust response criteria with near normalization of blood counts (n = 8). Post-EPAG iron measurements were performed in refSAA patients at a median of 12 months (range, 0.7-25.2 months) after discontinuation of the drug and at 2 months (range, 0.9-7.3 months) in MAA patients. In all of these patients, plasma iron concentrations significantly decreased after EPAG was stopped compared with the 6 month on-treatment value (off-treatment EPAG, 150.8 ± 19.5 µg/dL vs 6 months on-treatment EPAG, 441.6 ± 33.7 µg/dL; P = .002; Figure 1A). Multivariable analysis of variance suggested that changes in iron levels did not correlate with other patient characteristics, including age, sex, race, or transfusion status (supplemental Table 2).
Iron and ferritin concentrations in patients with aplastic anemia in relation to EPAG treatment. Longitudinal values of plasma iron (measured by colorimetric assay; reference range, 37-145 µg/dL in females and 59-158 µg/dL in males) and ferritin (measured by immunoassay; reference range, 13-150 µg/L in females and 30-400 µg/L in males) in (A) refSAA patients and (B) MAA patients before, during, and after EPAG treatment. RefSAA patients with iron studies available from baseline and at least one follow-up time point at 3 or 6 months of EPAG treatment were included in the analysis (38 of 40 enrolled patients). Selected MAA patients shown in panel B (n = 19) received no red blood cell transfusions on study (see supplemental Figure 1 for iron studies for the entire MAA study cohort). Posttreatment iron studies were performed at a median 12 months (range, 0.7-25.2 months) after EPAG was discontinued in refSAA patients and 2 months in MAA patients (range, 1.0-7.3 months). P values were calculated on the basis of paired Student t tests. ***P < .001; **P < .01; *P < .05. ns, not significant.
Iron and ferritin concentrations in patients with aplastic anemia in relation to EPAG treatment. Longitudinal values of plasma iron (measured by colorimetric assay; reference range, 37-145 µg/dL in females and 59-158 µg/dL in males) and ferritin (measured by immunoassay; reference range, 13-150 µg/L in females and 30-400 µg/L in males) in (A) refSAA patients and (B) MAA patients before, during, and after EPAG treatment. RefSAA patients with iron studies available from baseline and at least one follow-up time point at 3 or 6 months of EPAG treatment were included in the analysis (38 of 40 enrolled patients). Selected MAA patients shown in panel B (n = 19) received no red blood cell transfusions on study (see supplemental Figure 1 for iron studies for the entire MAA study cohort). Posttreatment iron studies were performed at a median 12 months (range, 0.7-25.2 months) after EPAG was discontinued in refSAA patients and 2 months in MAA patients (range, 1.0-7.3 months). P values were calculated on the basis of paired Student t tests. ***P < .001; **P < .01; *P < .05. ns, not significant.
We observed a similar increase in plasma iron levels in 19 MAA patients (baseline, 126.8 ± 10.2 µg/dL vs 4 months of EPAG treatment, 543.2 ± 62.3 µg/dL; n = 19; P < .001; Figure 1B) who did not receive further red blood cell transfusions after the start of EPAG treatment and were not phlebotomized or concurrently treated with iron chelators. As in patients with refSAA, plasma iron levels decreased back toward baseline after discontinuing the drug in 9 patients with MAA (159.4 ± 31.7 µg/dL; P = .003; see supplemental Figure 1 and supplemental Table 3 for patient characteristics and iron studies for the entire MAA cohort).
Ferritin levels did not change significantly between pretreatment and 3 or 6 months on EPAG in refSAA patients (Figure 1A) or in MAA patients (Figure 1B). In refSAA patients, ferritin levels were significantly lower after discontinuing EPAG compared with baseline (baseline, 2468.8 ± 326.0 µg/L vs off EPAG, 1018.3 ± 221.9 µg/L; P = .032; Figure 1A) but were comparable to pretreatment baseline values in evaluable MAA patients (Figure 1B). However, plasma serum ferritin levels were quite variable, and as an acute phase response protein, ferritin measurements lack specificity to detect subtle changes in the setting of normal or iron overloaded states. High ferritin levels in iron-overloaded patients are poorly correlated with the degree of total body iron stores.5,6
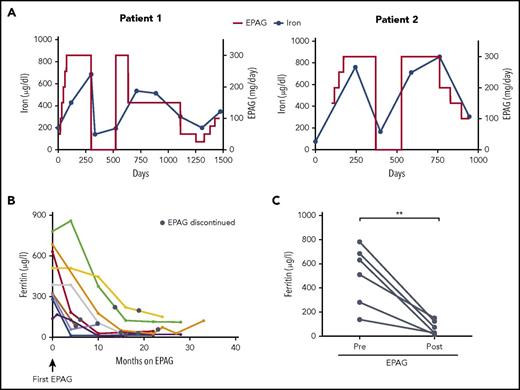
To investigate the relationship between EPAG and plasma iron concentrations, we analyzed sequential iron studies in 2 MAA patients in whom EPAG was discontinued intermittently as a result of reaching protocol criteria for robust response, but re-initiation of EPAG was required when blood counts later decreased (Figure 2A). In both cases, changes in plasma iron concentrations clearly increased and decreased coincident with receiving or not receiving EPAG and seemed to correlate with EPAG dosage. Of note, ferritin levels markedly decreased with prolonged EPAG treatment in both patients (supplemental Figure 2). Similarly, ferritin levels in responding MAA patients who received drug for more than 4 months continuously declined (Figure 2B) and were significantly lower compared with baseline values (Figure 2C), suggesting a relevant net iron loss with prolonged EPAG therapy.
Plasma iron and ferritin concentrations in aplastic anemia patients treated long term with EPAG. (A) Dose of EPAG administered and iron concentrations in 2 MAA patients from protocol initiation through current follow-up. EPAG was discontinued per protocol criteria for robust hematologic response on day 299 in patient 1 and on day 371 in patient 2. Subsequently, both patients experienced declining blood cell counts, and EPAG was re-initiated at the last effective dose per protocol. After achieving a second robust response, the EPAG dose was slowly tapered in both patients. Neither patient received concomitant iron chelators or red blood cell transfusions. (B) Ferritin levels in MAA patients with an EPAG treatment duration of at least 4 months. No patient received red blood cell transfusions, therapeutic phlebotomy, or pharmacological chelation during the time intervals graphed. The black circles indicate the time point of EPAG discontinuation. (C) Comparison of ferritin levels pretreatment and after EPAG discontinuation (first measurement after EPAG discontinuation corresponds to black circles in panel B) in 6 MAA patients with prolonged EPAG treatment and ferritin measurements available at follow-up. P value was calculated on the basis of paired Student t tests. **P = .005.
Plasma iron and ferritin concentrations in aplastic anemia patients treated long term with EPAG. (A) Dose of EPAG administered and iron concentrations in 2 MAA patients from protocol initiation through current follow-up. EPAG was discontinued per protocol criteria for robust hematologic response on day 299 in patient 1 and on day 371 in patient 2. Subsequently, both patients experienced declining blood cell counts, and EPAG was re-initiated at the last effective dose per protocol. After achieving a second robust response, the EPAG dose was slowly tapered in both patients. Neither patient received concomitant iron chelators or red blood cell transfusions. (B) Ferritin levels in MAA patients with an EPAG treatment duration of at least 4 months. No patient received red blood cell transfusions, therapeutic phlebotomy, or pharmacological chelation during the time intervals graphed. The black circles indicate the time point of EPAG discontinuation. (C) Comparison of ferritin levels pretreatment and after EPAG discontinuation (first measurement after EPAG discontinuation corresponds to black circles in panel B) in 6 MAA patients with prolonged EPAG treatment and ferritin measurements available at follow-up. P value was calculated on the basis of paired Student t tests. **P = .005.
Our data confirm the potential clinical relevance of previous in vitro studies reporting the iron-mobilizing properties of EPAG.4,5 This effect was achieved with clinically well-tolerated and US Food and Drug Administration–approved doses of EPAG. EPAG has been shown to interfere with some standard serum chemistry tests.6-8 However, as we previously reported, iron measurements were not affected by EPAG concentrations of up to 500 μg/mL,8 considerably above the levels likely reached in these clinical trials. To further investigate the potential for analytical interference, the accuracy of iron measurements was verified by analyzing serum iron concentrations in duplicate samples from a non-study patient who was receiving EPAG 150 mg/day by using an alternate, element-specific iron method (inductively coupled plasma/optical emission spectrometry) compared with the standard methodology used in the clinical laboratory (data not shown).
Our clinical trials were not designed to study the chelation properties of EPAG, and therefore we cannot answer the question of whether EPAG as a single agent or in combination with other chelators could be used to reduce total body iron. However, the reduction in ferritin levels in the MAA patients treated and observed over the long term and the recent case series reporting iron deficiency in 6 of 11 pediatric patients treated with EPAG for immune thrombocytopenia9 suggest a clinically meaningful EPAG-related net loss of iron and potential for the drug to be used as a chelator. EPAG is extensively metabolized in the liver, with 59% excreted in feces, of which only 20% is unchanged drug. Only EPAG metabolites can be detected in urine.10 Whether EPAG metabolites can chelate iron is unclear, and thus the amount of iron excreted in feces and urine while a patient is receiving EPAG is difficult to predict and is an important topic for future study. Vlachodimitropoulou et al5 elegantly showed more than additive iron mobilizing effects in vitro when EPAG was combined with other known chelators and proposed iron shuttling, in which one chelator donates an iron to a second as a possible mechanism for synergy.11
In conclusion, our study shows, for the first time, a clear relationship between increased plasma iron levels and EPAG treatment in patients. In addition, reduced ferritin levels in some patients receiving prolonged EPAG therapy suggest a clinically relevant chelation effect. Although there are many open questions regarding the mechanism of iron mobilization, distribution, and elimination, this observation warrants further investigation, with the aim of exploring whether EPAG alone or in combination could be used for iron chelation, particularly in patients with bone marrow failure. Furthermore, monitoring of iron studies should be considered in any patients treated with EPAG for prolonged periods.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, and by funding for clinical research from GlaxoSmithKline and Novartis (C.E.D., N.S.Y., D.M.T., and T.W.).
Authorship
Contribution: Z.Z. and Q.S. collected, assembled, and analyzed data and wrote the manuscript; L.J.S. and M.S. conceptualized the study; Z.C. analyzed data; S.G. collected and assembled data; D.M.T. and N.S.Y. provided study materials and edited the manuscript; C.E.D. conceptualized the study, analyzed and interpreted the data, and edited the manuscript; and T.W. conceptualized the study, collected, assembled, analyzed, and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cynthia E. Dunbar, Molecular Hematopoiesis Section, Hematology Branch, Building 10 CRC Room 4E-5132, National Heart, Lung and Blood Institute, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: dunbarc@nhlbi.nih.gov.
References
Author notes
Z.Z. and Q.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal