Key Points
Longer donor TL protects patients with SAA undergoing transplantation from infection-related death.
Abstract
Previous studies have suggested that longer donor leukocyte telomere length (TL) is associated with improved survival after hematopoietic cell transplantation (HCT) in severe aplastic anemia (SAA). This study aimed to determine whether cell-specific lymphocyte TL is associated with certain post-HCT causes of death. We used flow cytometry and fluorescence in situ hybridization to measure TL in donor total lymphocytes and subsets: naïve enriched T cells (CD45RA+CD20−), memory enriched T cells (CD45RA−CD20−), natural killer (NK) fully differentiated T cells (CD45RA+CD57+), and B cells (CD45RA+CD20+). Competing risk survival regression was used for cause-specific death analyses. Clinical data and biospecimens were available from the Center for International Blood and Marrow Transplant Research database and biorepository. The study included 197 patients who underwent unrelated-donor HCT for SAA between 1988 and 2004. The median age at HCT was 15 years (range, 0.5-40 years), and the median follow-up was 5 years (range, <1 month to 20.7 years). Longer donor TL in all cell subsets was associated with lower risk of all-cause mortality (P < .01). In cause-specific mortality analyses, longer TL in B cells (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.46-0.87; P = .006) and possibly NK fully differentiated T cells (HR, 0.7; 95% CI, 0.51 to 0.97; P = .03) was associated with lower risk of infection-related death. Donor TL in other tested lymphocyte subsets was not statistically significantly associated with death resulting from graft-versus-host disease or graft failure (P > .05). However, a trend toward excess risk of graft-versus-host mortality was noted (HR for total lymphocyte TL, 1.26; P = .15). In conclusion, longer donor TL was associated with reduced rate of infection-related deaths after HCT for SAA.
Introduction
Severe aplastic anemia (SAA) is a bone marrow failure disease caused by an immune-mediated process in most cases1 or inherited genetic mutations in a small subset of patients.2 Hematopoietic cell transplantation (HCT) is the first line of therapy for patients age <40 years with an available matched sibling donor; 3-year survival for those patients is 85%.3 For patients age >40 years or those lacking matching sibling donors, HCT is offered after failure of ≥1 cycle of immunosuppressive therapy. Survival after unrelated-donor HCT is improving but still associated with a relatively high mortality risk (3-year survival, 70%).3 The main causes of death after HCT in patients with SAA are infections, graft-versus-host disease (GVHD), and graft failure.4,5
Recent studies have shown an association with improved survival in patients with SAA undergoing unrelated HCT from donors with longer telomere length (TL).6,7 Telomeres consist of long tandem (TTAGGG)n repeats and a protein complex at the end of chromosomes. They maintain chromosomal stability by preventing end-to-end fusions. Telomeres shorten with every cell division and, as such, are markers of cellular replicative capacity and biological aging.8 Short telomeres in the general population are associated with mortality,9,10 age-related diseases, such as cardiovascular disease11 or cancer,12 and infections.13
The effect of donor TL on HCT outcomes for indications other than SAA is still unknown, but a recent study suggested no association between donor TL and outcomes in patients undergoing HCT for acute leukemia.14 This finding could be, in part, because donor TL has no effect on relapse-related mortality, the most common cause of death after HCT for malignant diseases. Furthermore, published studies of donor TL and HCT outcomes have relied on quantitative polymerase chain reaction (qPCR) measurement, which is limited by its sensitivity to preanalytic conditions15,16 and insufficient ability to detect short TL.17
Here, we reexamined the association between donor TL and post-HCT survival in patients with SAA using the highly accurate flow cytometry and fluorescence in situ hybridization (flow FISH) TL measurement. We also tested the effect of donor lymphocyte cell type–specific TL on the 3 main causes of post-HCT death in SAA: infections, GVHD, and graft failure.
Methods
From the Center for International Blood and Marrow Transplant Research (CIBMTR) database and biorepository, we identified 217 patients who underwent unrelated HCT for SAA and for whom a donor cryopreserved peripheral blood mononuclear cell sample and outcome data were available. Samples were collected and cryopreserved by CIBMTR only before 2005. CIBMTR is a research collaboration between the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin. CIBMTR collects HCT clinical and outcome data on every allogeneic transplantation performed in the United States and voluntary data contribution from transplantation centers around the world. Causes of death were assigned by transplantation centers. All patients provided informed consent for participation in the CIBMTR database and biorepository. This study was approved by the National Marrow Donor Program Institutional Review Board and the National Institutes of Health Office of Human Subjects Research.
TL measurement
We used flow FISH and measured TL in donor total lymphocytes and subsets: naïve enriched T cells (CD45RA+CD20−), memory enriched T cells (CD45RA−CD20−), natural killer (NK) fully differentiated T cells (CD45RA+CD57+), and B cells (CD45RA+CD20+). A detailed description of the method is available elsewhere.18 Briefly, cryopreserved peripheral blood mononuclear cells from HCT donors were washed and then mixed with bovine thymocytes of known TL (internal control), denatured in formamide at 87°C, hybridized with a fluorescein-conjugated (CCCTAA)3 peptide nucleic acid probe specific for telomere repeats, and counterstained with LDS751 DNA dye. Lymphocytes were identified based on light scatter and fluorescence intensity and further characterized into subsets defined by labeled antibodies specific for CD20, CD45RA, and CD57 relative to internal control cells. TLs for total lymphocytes and for cell subtypes were measured for 203 donors; 6 were excluded because they had donated peripheral blood stem cells. All patients included in this study were also part of our original study, in which we used qPCR assay for TL measurement.7 Laboratory personnel were blinded to patient outcomes and qPCR TL results.
Statistical analysis
We used Cox proportional hazards models to evaluate all-cause mortality in relation to donor total and cell type–specific lymphocyte TL. For cause-specific mortality analyses, we used Fine and Gray’s proportional subdistribution hazards models to account for competing risk associated with death resulting from causes other than that under investigation. We focused on death resulting from infections, GVHD, and graft failure, because these are the most common causes of post-HCT mortality in SAA. We constructed separate models by cell type–specific TL. A P value of .01 (.05/5) was considered statistically significant to account for multiple comparisons across total lymphocytes and the 4 cell subtypes. Clinical variables included in the models were selected via a backward stepwise selection procedure with a P threshold of .05 for entry and .1 for removal. Additionally, all models were adjusted for donor age. Follow-up time started at the date of HCT and ended at death or was censored at date of last follow-up or end of study on 22 March 2013.
Results
The study included 197 patients with SAA who received unrelated-donor bone marrow transplants between 1988 and 2004 at a median age of 15 years (range, 0.5-40 years). A majority had acquired disease (acquired SAA, 67.5%; Fanconi anemia, 28.9%; and Diamond-Blackfan anemia, 3.6%), approximately half were male (n = 104; 54.7%), and half received myeloablative conditioning regimens (n = 98; 49.7%). The median donor age was 37 years (range, 20-55 years; Table 1). The median follow-up time was 5 years (range, <1 month to 20.7 years)
Demographic and clinical characteristics of patients with SAA in the study
| Variable . | N (%) . |
|---|---|
| No. of patients | 197 |
| Recipient age, y | |
| Median | 15 |
| Range | 0.5-40 |
| Donor age, y | |
| Median | 37 |
| Range | 20-55 |
| Male sex | 104 (54.7) |
| Disease subtype | |
| Acquired SAA | 133 (67.5) |
| Fanconi anemia | 57 (28.9) |
| Diamond-Blackfan anemia | 7 (3.6) |
| Myeloablative regimens | 98 (49.7) |
| White race | 149 (75.6) |
| Karnofsky performance status 90-100 | 151 (76.6) |
| Year of HCT | |
| 1988-1995 | 63 (33.2) |
| 1996-1999 | 73 (38.4) |
| 2000-2004 | 54 (28.4) |
| Died during follow-up | 135 (68.5) |
| Cause of death | |
| Infection | 35 |
| Acute or chronic GVHD | 24 |
| Primary or secondary graft failure | 14 |
| Other causes* | 62 |
| Variable . | N (%) . |
|---|---|
| No. of patients | 197 |
| Recipient age, y | |
| Median | 15 |
| Range | 0.5-40 |
| Donor age, y | |
| Median | 37 |
| Range | 20-55 |
| Male sex | 104 (54.7) |
| Disease subtype | |
| Acquired SAA | 133 (67.5) |
| Fanconi anemia | 57 (28.9) |
| Diamond-Blackfan anemia | 7 (3.6) |
| Myeloablative regimens | 98 (49.7) |
| White race | 149 (75.6) |
| Karnofsky performance status 90-100 | 151 (76.6) |
| Year of HCT | |
| 1988-1995 | 63 (33.2) |
| 1996-1999 | 73 (38.4) |
| 2000-2004 | 54 (28.4) |
| Died during follow-up | 135 (68.5) |
| Cause of death | |
| Infection | 35 |
| Acute or chronic GVHD | 24 |
| Primary or secondary graft failure | 14 |
| Other causes* | 62 |
Other causes of death include: hemorrhage, n = 16; acute respiratory distress syndrome or interstitial pneumonitis, n = 13; toxicity, n = 10; organ failure, n = 8; cancers, n = 3; and missing, n = 12.
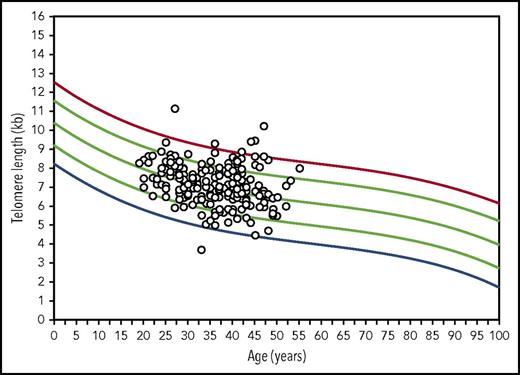
The median donor total lymphocyte TL was 7.1 kb (range, 3.7-11.2 kb). TL in lymphocyte cell subsets was longest in B cells (median, 8.2 kb; range, 5.4-11.5 kb) and shortest in NK (median, 6.1 kb; range, 3.3-10.1 kb) and memory cells (median, 6.2 kb; range, 3.4-10.9 kb). The correlations between total lymphocyte TL and cell type–specific TL was high for naïve and memory T-cell TL (r = 0.92 and 0.93, respectively; P < .001) and modest for B-cell and NK cell TL (r = 0.77 and 0.79, respectively; P < .001; Table 2). When donor TL was plotted on curves generated from 400 healthy volunteers, lymphocyte TL was normal for age in a majority of donors (between the 10th and 90th percentiles; n = 153; 77.7%); 16 donors had short lymphocyte TL (<10th percentile for age); 1 had TL < first percentile for age; 28 donors had long TL (>90th percentile for age; Figure 1). As expected, all TL measurements were negatively correlated with donor age; the correlation coefficient was highest for naïve T-cell TL (r = −0.34; P < .001) and lowest for memory T-cell (r = −0.16; P < .01) and B-cell TL (r = −0.18; P < .001; Table 2).
Donor lymphocyte TL and correlations with age- and cell-specific TL values
| Cell type . | Median (range) TL, kb . | Correlation coefficient r (P) . | |
|---|---|---|---|
| Age . | Total lymphocyte TL . | ||
| Total lymphocyte | 7.1 (3.7-11.2) | −0.25 (<.001) | 1 |
| Naïve enriched T cells (CD45RA+CD20−) | 7.8 (4.0-11.4) | −0.34 (<.001) | 0.92 (<.001) |
| Memory enriched T cells (CD45RA−CD20−) | 6.2 (3.4-10.9) | −0.16 (.02) | 0.79 (<.001) |
| NK fully differentiated T cells (CD45RA+CD57+) | 6.1 (3.3-10.1) | −0.24 (<.001) | 0.79 (<.001) |
| B cells (CD45RA+CD20+) | 8.2 (5.4-11.5) | −0.18 (<.001) | 0.77 (<.001) |
| Cell type . | Median (range) TL, kb . | Correlation coefficient r (P) . | |
|---|---|---|---|
| Age . | Total lymphocyte TL . | ||
| Total lymphocyte | 7.1 (3.7-11.2) | −0.25 (<.001) | 1 |
| Naïve enriched T cells (CD45RA+CD20−) | 7.8 (4.0-11.4) | −0.34 (<.001) | 0.92 (<.001) |
| Memory enriched T cells (CD45RA−CD20−) | 6.2 (3.4-10.9) | −0.16 (.02) | 0.79 (<.001) |
| NK fully differentiated T cells (CD45RA+CD57+) | 6.1 (3.3-10.1) | −0.24 (<.001) | 0.79 (<.001) |
| B cells (CD45RA+CD20+) | 8.2 (5.4-11.5) | −0.18 (<.001) | 0.77 (<.001) |
Scatter plots of donor flow FISH lymphocyte TL represented on age-matched control curves. Lines represents the first, 10th, 50th, 90th, and 99th percentiles of TL for ages 0 to 100 years constructed from 400 healthy individuals.
Scatter plots of donor flow FISH lymphocyte TL represented on age-matched control curves. Lines represents the first, 10th, 50th, 90th, and 99th percentiles of TL for ages 0 to 100 years constructed from 400 healthy individuals.
Models for post-HCT all-cause mortality showed statistically significant associations with total lymphocyte TL and with each lymphocyte cell subset. Each 1-kb increase in total lymphocyte donor TL was associated with 23% longer survival (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.64-0.92; P = .004). Analysis stratified by disease subtype showed similar results. In patients with inherited SAA, the HR was 0.67 (95% CI, 0.46-0.98; P = .04); in patients with acquired SAA, the HR was 0.79 (95% CI, 0.65-0.97; P = .03). For cell type–specific TL, the association with B-cell TL was the strongest (HR, 0.73; 95% CI, 0.61-0.88; P = .001; Table 3). Donor age was not statistically significantly associated with patient overall survival in any of the TL models (P < .1).
Associations between donor lymphocyte TL and all-cause and cause-specific mortality after unrelated-donor HCT for SAA
| . | HR (95% CI)*P . | |||
|---|---|---|---|---|
| All-cause mortality . | Infection mortality . | GVHD mortality . | Graft failure mortality . | |
| Total lymphocyte | 0.77 (0.64-0.92) .004 | 0.81 (0.59-1.10) .10 | 1.26 (0.92-1.72) .15 | 0.95 (0.54-1.70) .88 |
| Naïve enriched T cells | 0.81 (0.69-0.95) .009 | 0.84 (0.63-1.14) .26 | 1.20 (0.89-1.61) .23 | 0.82 (0.47-1.43) .49 |
| Memory enriched T cells | 0.77 (0.64-0.93) .008 | 0.72 (0.51-0.97) .06 | 1.11 (0.80-1.55) .52 | 0.95 (0.55-1.63) .84 |
| NK fully differentiated T cells | 0.77 (0.65-0.93) .005 | 0.70 (0.51-0.97) .03 | 1.20 (0.80-1.82) .37 | 0.86 (0.51-1.40) .56 |
| B cells | 0.73 (0.61-0.88) .001 | 0.63 (0.46-0.87) .006 | 1.10 (0.76-1.59) .60 | 1.12 (0.67-1.86) .67 |
| . | HR (95% CI)*P . | |||
|---|---|---|---|---|
| All-cause mortality . | Infection mortality . | GVHD mortality . | Graft failure mortality . | |
| Total lymphocyte | 0.77 (0.64-0.92) .004 | 0.81 (0.59-1.10) .10 | 1.26 (0.92-1.72) .15 | 0.95 (0.54-1.70) .88 |
| Naïve enriched T cells | 0.81 (0.69-0.95) .009 | 0.84 (0.63-1.14) .26 | 1.20 (0.89-1.61) .23 | 0.82 (0.47-1.43) .49 |
| Memory enriched T cells | 0.77 (0.64-0.93) .008 | 0.72 (0.51-0.97) .06 | 1.11 (0.80-1.55) .52 | 0.95 (0.55-1.63) .84 |
| NK fully differentiated T cells | 0.77 (0.65-0.93) .005 | 0.70 (0.51-0.97) .03 | 1.20 (0.80-1.82) .37 | 0.86 (0.51-1.40) .56 |
| B cells | 0.73 (0.61-0.88) .001 | 0.63 (0.46-0.87) .006 | 1.10 (0.76-1.59) .60 | 1.12 (0.67-1.86) .67 |
Regarding HR and 95% CI, models were adjusted for: all cause-mortality: donor age, HLA matching, disease subtype, HCT year, prior SAA therapy, and recipient race; infection-related death: donor age, HLA matching, disease subtype, Karnofsky performance status (KPS), year of HCT, and prior SAA therapy; GVHD-related death: donor age, HLA matching, disease subtype, KPS, year of HCT, prior SAA therapy, and GVHD prophylaxis; and graft failure death: donor age, HLA matching, disease subtype, KPS, year of HCT, and prior SAA therapy.
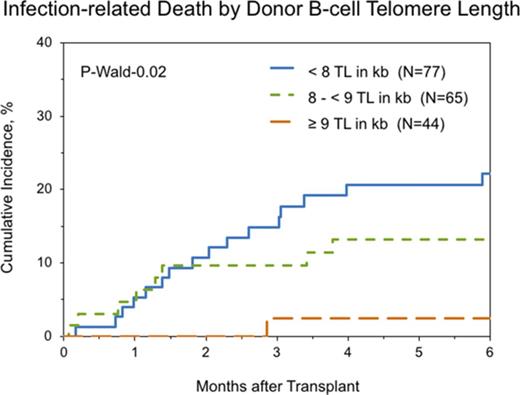
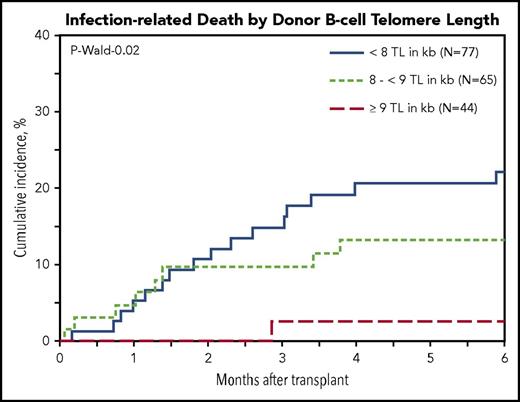
Multivariable cause-specific mortality analyses showed statistically significant associations between longer B-cell TL and lower risk of infection-related death (HR, 0.63; 95% CI, 0.46-0.87; P = .006; Figure 2). The data also suggested possible associations with longer TL of NK-differentiated T cells (HR, 0.81; 95% CI, 0.67-0.98; P = .03), although the study threshold for statistical significance (P < .01) was not met.
Cumulative incidence of posttransplantation infection-related death in patients with SAA by donor B-cell TL.
Cumulative incidence of posttransplantation infection-related death in patients with SAA by donor B-cell TL.
No statistically significant associations were noted between donor TL in any of the investigated cell types and death resulting from acute or chronic GVHD or primary or secondary graft failure (P > .05 in all); however, the data suggested a possible association with GVHD mortality (HR for total lymphocytes, 1.26; range for all T-cell subsets, 1.1-1.2; P > .1 in all; Table 3).
Discussion
Telomeres are markers for cellular replicative capacity and biological aging. Our flow FISH TL data confirmed previous findings of improved posttransplantation survival associated with receiving transplants from donors with long average leukocyte TL measured by qPCR. Longer TL in donor B cells (HR, 0.61; P = .002) and possibly in NK-differentiated T cells (HR, 0.70; P = .03) was associated with lower risk of infection-related death after HCT for acquired and inherited SAA. In neither total lymphocytes nor lymphocyte subsets was donor TL statistically significantly associated with risk of post-HCT mortality resulting from GVHD or graft failure.
The current study results are in agreement with our previous findings of a survival advantage associated with receiving transplants from a donor with longer leukocyte TL.6,7 The use of the highly accurate flow FISH assay for TL measurement in the current evaluation showed a stronger association between donor TL and recipient post-HCT survival. Our previous study of qPCR TL measurement in 300 donors (66% were part of the current analysis) found the survival benefit was restricted to donors with TLs in the longest tertile of TL. Here, we show that the association is linear, with a 23% reduction in mortality risk for every 1-kb increase in donor lymphocyte TL. The correlations between flow FISH and qPCR TL were modest in several studies.19,20
Selecting young donors for allogeneic HCT is the current clinical practice, supported by findings from several large studies.21,22 Most recently, a study including >10 000 patients undergoing HCT showed an approximately 6% increase in the hazard of dying after transplantation with every 10-year addition in donor age.22 Here, we show that donor TL association with post-HCT survival is independent of age, because all models were adjusted for donor age. TL is highly variable among individuals, with an approximately 4-kb range difference in adults of similar age23 (this is equivalent to ≥40 years in chronological age). This is supportive of the concept that chronological age is an imprecise measure of cellular aging. Consequently, our data support the use of donor TL as a marker for donor selection instead of age for recipients of unrelated-donor transplants for aplastic anemia.
Similar to results from a larger flow FISH study of >800 individuals,23 our data showed differences in TL among lymphocyte cell types, where B cells followed by naïve T cells had the longest TLs and memory T cells and NK-differentiated cells had the shortest. This may be a result, in part, of age-related telomere dynamics between B- and T-cell subpopulations, with B cells showing the lowest rate of attrition over time.24,25 In the current study, B-cell TL provides an explanation for the observed survival advantage associated with longer donor TL, with a 37% reduction in infection-related deaths with each 1-kb increase in donor TL. This is in agreement with results from a previous study of individuals age ≥60 years showing a strong association between shorter blood TL and risk of subsequent mortality resulting from infections (RR, 8.5; P = .01).9 Similarly, a recent prospective study of 75 309 individuals from Denmark showed an association between shorter leukocyte TL and risk of infection-related hospitalization and death.13 Longer TL in immune cells is an indication of better immune function. Najarro et al26 showed that longer B-cell TL was associated with a fourfold increase in vaccine-specific antibody titers measured at day 21 or 84 after influenza vaccination.
In agreement with our previous study showing no association between donor TL and GVHD or post-HCT hematological recovery,7 here we show no association with death resulting from GVHD or graft failure. The null association between donor TL and graft failure is in contrast to reports of secondary graft failure in patients with inherited telomere biology defects, where telomeres are usually very short.27 The discrepancy can be explained by the observed range of TLs in the donors of this study, where almost all had TLs within the normal range despite significant variation.
Results from this study suggest a possible explanation for the lack of association between donor TL and survival in patients with acute leukemia.14 It is possible that the donor TL effect in leukemia is obscured by the relatively lower contribution of infection-related death to post-HCT all-cause mortality. Using data from the European Group for Blood and Marrow Transplantation, Gratwohl et al28 showed that the 5-year cumulative incidence of death resulting from relapse was 12% as compared with 5% for infection-related mortality in a cohort of 14 403 patients receiving matched-sibling transplants for early leukemia.28 Analysis of cause-specific deaths according to cell-specific TL may identify previously unappreciated roles of donor TL in HCT outcomes for patients with leukemia.
Our novel findings related to all-cause mortality showed a statistically significant association with each TL lymphocyte subset (P < .01 for all). This indicates that TL may play a role in causes of post-HCT mortality other than those examined in this study, with NK cells seeming to have the strongest association (HR for death resulting from causes other than those tested, 0.69; P = .007). The small sample size for each of the other causes did not allow for further investigation. The study was limited by restriction of the transplantation period to between 1988 and 2004; the CIBMTR sample collection of cryopreserved peripheral blood mononuclear cells was limited to this period.
In conclusion, our study highlights a potentially important role for selecting donors with longer telomeres to prevent infection-related death. A prospective larger study is needed to validate these finding in light of the recent improvement in HCT outcomes for patients with SAA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), National Institutes of Health; by a public health service grant (5U24CA076518) from the National Institutes of Heath, NCI, the National Heart Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; by a grant/cooperative agreement (4U10HL069294) from the NHLBI and NCI; by a contract from the Health Resources and Services Administration (HHSH234200637015C); and by grants N00014-17-1-2388, N00014-16-1-2020, and N0014-17-1-2850 from the Office of Naval Research.
Authorship
Contribution: S.M.G., S.A.S., S.R.S., and S.J.L. designed the study; M.H. and S.R.S. acquired the data; G.A. performed telomere length measurements and analysis; S.M.G., M.H., L.W., H.A.K., and T.W. analyzed the data; all authors interpreted the data; S.M.G. wrote the manuscript; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: G.A. holds part-time employment with Repeat Diagnostics Inc., a company specializing in clinical telomere length measurements. The remaining authors declare no competing financial interests.
Correspondence: Shahinaz M. Gadalla, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, 9609 Medical Center Dr, Room 6E-534, Rockville, MD 20850; e-mail: gadallas@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal