Abstract
Myofibroblasts are fibrosis-driving cells and are well characterized in solid organ fibrosis, but their role and cellular origin in bone marrow fibrosis remains obscure. Recent work has demonstrated that Gli1+ and LepR+ mesenchymal stromal cells (MSCs) are progenitors of fibrosis-causing myofibroblasts in the bone marrow. Genetic ablation of Gli1+ MSCs or pharmacologic targeting of hedgehog (Hh)-Gli signaling ameliorated fibrosis in mouse models of myelofibrosis (MF). Moreover, pharmacologic or genetic intervention in platelet-derived growth factor receptor α (Pdgfrα) signaling in Lepr+ stromal cells suppressed their expansion and ameliorated MF. Improved understanding of cellular and molecular mechanisms in the hematopoietic stem cell niche that govern the transition of MSCs to myofibroblasts and myofibroblast expansion in MF has led to new paradigms in the pathogenesis and treatment of MF. Here, we highlight the central role of malignant hematopoietic clone-derived megakaryocytes in reprogramming the hematopoietic stem cell niche in MF with potential detrimental consequences for hematopoietic reconstitution after allogenic stem cell transplantation, so far the only therapeutic approach in MF considered to be curative. We and others have reported that targeting Hh-Gli signaling is a therapeutic strategy in solid organ fibrosis. Data indicate that targeting Gli proteins directly inhibits Gli1+ cell proliferation and myofibroblast differentiation, which results in reduced fibrosis severity and improved organ function. Although canonical Hh inhibition (eg, smoothened [Smo] inhibition) failed to improve pulmonary fibrosis, kidney fibrosis, or MF, the direct inhibition of Gli proteins ameliorated fibrosis. Therefore, targeting Gli proteins directly might be an interesting and novel therapeutic approach in MF.
Introduction
Under normal conditions, the bone marrow provides a fine network of fibers that provides support for the blood cells to grow. Bone marrow fibrosis or myelofibrosis (MF) is a disorder in which normal bone marrow tissue and blood-forming cells are gradually replaced with thick coarse fibers and scar-like tissue.1 By definition, MF implies an increase in the bone marrow fiber content without explicit reference to quantity or quality. Over time, this leads to failure of the bone marrow to produce blood cells and to extramedullary hematopoiesis (typically in the spleen) resulting in hepatosplenomegaly and ultimately in death. MF is a concomitant and nonspecific feature that is a result of a multitude of reactive as well as neoplastic disorders.
The myeloproliferative neoplasm (MPN) primary MF (PMF), previously known as chronic idiopathic MF or agnogenic myeloid metaplasia, is the prototypic example of progressive development of bone marrow fibrosis, has the worst prognosis of MPNs, and is a complex disorder. Pathologically, MF is characterized by thickening and distortion of bony trabeculae, deposition of reticulin and collagen fibers, and megakaryocytic hyperplasia with atypical features.
In an editorial in Blood in 1951, Dameshek2 speculated that in MPNs, the bone marrow proliferates excessively as a unit in response to a myelostimulatory factor. He believed that this concept could explain why the hyperplasia in MPNs includes all myeloid lineages and, because fibrosis is often present, the fibroblasts as well. It has become increasingly clear over the last couple of years that 2 distinct pathogenic processes contribute to the initiation and progression of PMF: stem cell–derived clonal myeloproliferation and a reactive cytokine-driven inflammatory fibrosis. A simplified description of this process states that in PMF (or in MPNs in general), hematopoietic stem cells (HSCs) acquire mutations, in most of the cases a JAK2V617F mutation, which leads to increased proliferation of the HSC and replacement of normal blood formation.3-8 In the course of the disease, nonmutated, nonhematopoietic cells transform into fibrosis-driving cells. The biology of this cross talk between malignant hematopoietic cells and a normal (nonhematopoietic) stromal cell that transforms into a fibrosis-driving cell remained elusive for decades; the cells driving fibrosis were just identified in 2017.9,10 The discovery of Gli1+ and leptin receptor+ (LepR+) cells opened new avenues for dissecting pathomechanisms underlying the fibrotic transformation and identifying novel therapeutic targets for this so far incurable disease. Interestingly, these findings depicted striking similarities in the pathogenesis of solid organ fibrosis and MF. In particular, it became obvious that the common denominator of Gli1+ and LepR+ stromal cells is their progenitor state of fibrosis-causing myofibroblasts in the bone marrow. Myofibroblasts are fibrosis-driving cells, and they are well characterized in solid organ fibrosis, but their role and cellular origin in bone marrow fibrosis has remained obscure. Understanding the cellular and molecular mechanisms that govern the transition of mesenchymal stromal cells (MSCs) to myofibroblasts and myofibroblast expansion in the bone marrow will be critical to understanding the pathogenesis of bone marrow fibrosis and will guide the development of novel therapeutics. In this perspective article, we will summarize the recent discoveries of MSCs as myofibroblast precursors and their implications for therapeutic options in the context of knowledge of the pathogenesis and therapy of solid organ fibrosis.
Stromal cells as the cellular origin of myofibroblasts
Chronic repetitive injury leads to fibrosis in virtually any organ. It has been estimated that fibrosis is involved in up to 45% of all deaths in the developed world.11 The replacement of functional units of cells by myofibroblasts and extracellular matrix leading to progressive loss of organ function is the common feature of fibrosis across all organs. In solid organ fibrosis, researchers across different disciplines have agreed that myofibroblasts are the cells that drive fibrosis. Myofibroblasts are highly synthetically active and contractile cells that are characterized by dense rough endoplasmatic reticulum, collagen secretion granules, and α-smooth muscle actin (α-SMA) expression.12 α-SMA forms characteristic strong bundles of myofilaments, the so-called stress fibers that promote strong contractile force generation and that have been widely used to identify myofibroblasts in fibrotic tissue. Multiple genetic fate tracing experiments have shed light on the cellular origin of myofibroblasts in heart, kidney, liver, lung, and skin.13-15 More than 25 years ago, investigators observed myofibroblasts in electron microscopy studies of human bone marrow fibrosis,16,17 but their functional role and contribution to bone marrow fibrosis remained unclear. The literature on myofibroblasts in most organ systems is extensive, but, to the best of our knowledge, there have been no other studies that have followed up on this finding or investigated the origin and role of myofibroblasts in bone marrow fibrosis.
We recently identified that expression of the hedgehog (Hh) transcriptional activator Gli1 specifically marks perivascular MSC-like cells.14 Genetic fate tracing in heart, kidney, liver, and lung injury demonstrated that these cells are a major source of myofibroblasts during solid organ fibrosis.14 This prompted us to investigate the role of Gli1+ MSC-like cells in MF. In the bone marrow, Gli1+ cells are located in the endosteal niche and have direct contact with bone as well as in the perivascular niche around arterioles and marrow sinusoids.9 Inducible genetic fate tracing experiments in 2 murine models of bone marrow fibrosis (thrombopoietin [TPO] or JAK2V617F overexpression in HSCs) indicated that Gli1+ cells are fibrosis-driving α-SMA+ myofibroblasts of the bone marrow.9 By using heritable expression of the human diphtheria toxin receptor in Gli1+ cells, we demonstrated that ablation of Gli1+ cells abolishes bone marrow fibrosis. These data indicated that Gli1+-derived myofibroblasts are the cellular source of bone marrow fibrosis and are a promising therapeutic target.
Decker et al10 recently performed noninducible genetic fate tracing in LepR-Cre;tdTomato;Col1a1-GFP mice that received TPO-overexpressing bone marrow and demonstrated that LepR+stromal cells expand tremendously and become collagen-producing myofibroblasts in bone marrow fibrosis. They also demonstrated that LepR+ cells express the mesenchymal markers platelet-derived growth factor receptor α (PDGFRα) and PDGFRβ (also known as CD140a+b) and that PDGFRα is critical in the development of bone marrow fibrosis because a LepR+ cell–specific knockout of PDGFRα resulted in greatly reduced reticulin fibrosis.10 These 2 recent studies confirm, for the first time, the longstanding hypothesis that MSCs are indeed the cellular origin of bone marrow fibrosis. In addition to the above-mentioned studies on Gli1+ cells in solid organ fibrosis, seemingly equivalent tissue-resident MSC populations have been reported as the cellular drivers of organ fibrosis in various organ systems.18 It remains elusive whether Gli1+ and LepR+ cells overlap or are distinct myofibroblast precursors in the bone marrow. The role of monocyte-derived fibroblasts was recently discussed in MF.19 Gli1+ and LepR+ cells are CD45– nonhematopoietic cells, which indicates that their differentiation into myofibroblasts is a distinct process and is independent of monocyte-derived fibrocytes. Although fate tracing indicates that both stromal populations contribute to the myofibroblast pool, the data also suggest that not all myofibroblasts are derived from these stromal populations. Thus, contribution of other cell types to the bone marrow myofibroblast pool remains an open question, and monocyte-derived myofibroblasts might also contribute. More widely available technologies for single-cell analysis (eg, single-cell RNA sequencing) will shed light on the heterogeneity of the bone marrow stroma in homeostasis and disease.
Recent data indicate that in addition to eliminating the malignant hematopoietic clone, targeting the mesenchymal stroma might be a novel therapeutic strategy in MF. To explore whether pharmacologically targeting the Hh-Gli signaling pathway might affect expansion of Gli1+ stromal cells and fibrosis severity, we combined genetic fate tracing of Gli1+ cells in the JAK2V617F-induced MF model with a pharmacologic therapeutic regimen using the small-molecule direct Gli inhibitor Gli antagonist 61 (GANT61). Treatment with GANT61 or vehicle was initiated 8 weeks after bone marrow transplantation. Pharmacologic Gli inhibition resulted in greatly decreased Gli1+ cell expansion and virtually abolished bone marrow fibrosis.9 In addition, treatment with GANT61 reduced splenomegaly along with the JAK2V617F-induced hematopoietic phenotype with increased long-term HSCs and multipotent progenitor cells, which suggests an effect of both the malignant clone and fibrosis-driving stromal cells.9
We and others reported that targeting Hh-Gli signaling is a therapeutic strategy in solid organ fibrosis. Indeed, the data indicate that targeting Gli proteins directly inhibits Gli1+ cell proliferation and myofibroblast differentiation, which results in reduced fibrosis severity and improved organ function.20 Although inhibition of Hh-Gli signaling upstream (eg, by smoothened [Smo] inhibition) failed to improve pulmonary fibrosis21 and kidney fibrosis,22 the direct inhibition of Gli proteins by GANT61 ameliorated fibrosis in both organs.20,21 Therefore, targeting Gli proteins directly might be an interesting and novel therapeutic approach in MF.
The treatment of MF can be divided into 2 broad categories: eradication of the malignant hematopoietic clone and targeting of various signaling pathways and mediators implicated in MF. In MF, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only therapeutic intervention with curative potential, effectively eradicating the malignant hematopoietic clone and leading to reversal of fibrosis and MPN-associated histomorphologic features of the bone marrow.23 Importantly, regression of bone marrow fibrosis at day +100 after HSCT in patients with MF is associated with improved survival, independent of the International Prognostic Scoring System (IPSS) score at the time of transplantation, as demonstrated by Kröger et al.24 This study highlights the prognostic significance of bone marrow fibrosis, which is now accounted for in the Mutation-Enhanced IPSS for transplantation-age patients with MF.25 It also increases the potential for improving HSCT outcomes by combining antifibrogenic strategies before or even after the transplantation.
Development of therapeutic strategies in MF
Apart from HSCT, none of the conventional therapies used to treat MF are considered curative. With the introduction of selective JAK inhibitors over the last 10 years, initial expectation of clonal suppression was dampened by the observations of persistent clonal hematopoiesis and mostly unaltered MPN morphologic features in the bone marrow.26-28 One publication and several abstracts on more detailed histopathologic analyses of the bone marrow indicate that treating MF with ruxolitinib modulates the microenvironment because it reduces the number of macrophages and mast cells, microvascular density, microvessel area, and the inflammatory reaction. Long-term therapy with ruxolitinib may thus provide a clinically meaningful delay in the progression of bone marrow fibrosis because the observed alterations in the microenvironment seem to be associated with more favorable outcome.29-32 Furthermore, JAK inhibitors have been shown to improve patient constitutional symptoms and reduce splenomegaly, but the monotherapy does not significantly reduce mutant allele burden in the majority of MPN patients.28,33,34 The therapeutic window for JAK inhibitors is limited because of the essential role of the JAK/STAT signaling pathway in normal hematopoiesis, which has been observed in the clinic where these inhibitors have been associated with dose-limiting toxicities. Thus, there is a need to identify additional pathways that might be involved in the development and maintenance of MPN-mutant clones, which could be targeted in combination with JAK inhibition for improved therapeutic benefit.35-37
Identifying novel and effective antifibrotic therapeutics that have minor adverse effects in both solid organ fibrosis and MF is the holy grail in fibrosis research. Various antifibrotic strategies in solid organs and MF aim to target inflammation and fibrogenic cytokines, such as PDGF and transforming growth factor β (TGF-β), among various others.11,13 Targeting fibrogenic cytokines alone or in combination with ruxolitinib is an active area of investigation in the treatment of MF. Pirfenidone, the first antifibrotic compound on the market (approved in Japan for idiopathic pulmonary fibrosis in 2008, in Europe in 2011, and in the United States in 2014 by the US Food and Drug Administration), has anti-inflammatory properties and inhibits TGF-β and p38 signaling.38 The results of a phase 2 trial of pirfenidone treatment in 28 MF patients were published in 2001.39 Although TGF-β and inflammation are thought to play a central role in the disease pathogenesis of MF, none of the patients had improvement in bone marrow fibrosis or anemia or a reduction in spleen weight. Other strategies focus on resolving the excessive formation of extracellular matrix in fibrosis. Lysyl oxidase (LOX)-like oxidases are amine oxidases involved in the crosslinking of collagen and elastin fibers and were described as being upregulated in the serum of patients with MF.40 Simtuzumab, a humanized antibody against LOX-like 2, was tested in monotherapy and in combination with ruxolitinib. Simtuzumab alone or with ruxolitinib was well tolerated but did not produce clinical benefit or consistently reduce bone marrow fibrosis by 24 weeks.41 Human pentraxin 2 (PTX2) is a soluble pattern recognition receptor of the innate immune system that may aid in the removal of damaged tissue and regulate monocyte differentiation states.42 PRM-151, a recombinant form of PTX2, was investigated in treatment of various fibrotic diseases42 and MF, alone and in combination with ruxolitinib. A phase 2 study of PRM-151 in combination with ruxolitinib (13 patients completed at least 72 weeks) showed that the combination was well tolerated, and the authors reported an infrequent improvement in fibrosis grade at week 48.43 However, long-term results need to be assessed before final conclusions can be drawn.
In summary, results from these studies suggest that targeting single inflammatory cytokines or extracellular matrix proteins alone might not be sufficient for inhibiting fibrotic transformation but that multiple pathways that drive cellular processes in the fibrotic transformation need to be inhibited in parallel to block the complex interaction among malignant hematopoietic cells, inflammatory cytokines, dysplastic megakaryocytes, and fibrosis-driving cells.
Targeting Hh-Gli signaling as a novel therapeutic option
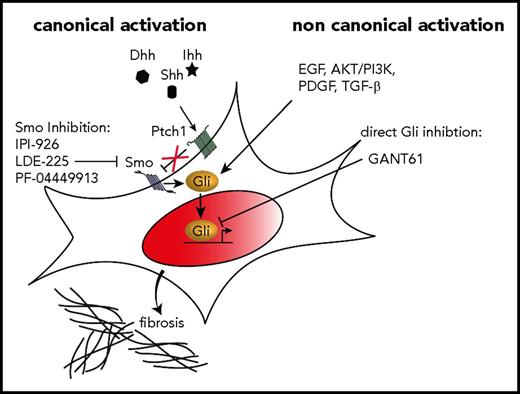
A 20- to 100-fold increase in the expression of Hh target genes, including Gli1 and Ptch1, has been observed in granulocytes isolated from MPN patients44 as well as an increased expression in the spleen of GATA1low myelofibrotic mice.45 Preclinical and clinical data suggest that Hh pathway inhibitors have therapeutic activity in MF. Sonidegib (LDE-225), saridegib (IPI-926), and PF-04449913, all Smo inhibitors, are currently under clinical investigation in MF, alone or in combination with other agents. The essential function and drugability render Smo well suited for pharmacologic inhibition. Sonidegib in combination with ruxolitinib was tested in a multicenter, phase 1b/2 study; the maximum tolerated dose was not reached during the dose-escalation phase, but improvements in leukocytosis, thrombocytosis, bone marrow fibrosis, and mutant allele burden were noticed, which indicates synergistic effects of sonidegib and ruxolitinib.46 A total of 27 MF patients were treated with ruxolitinib and sonidegib at the recommended phase 2 dose. Although the combination was relatively well tolerated and resulted in approximately 50% of patients achieving a ≥35% decrease in spleen volume, only 2 treated patients had a reduction of at least 1 grade in bone marrow fibrosis. A clinical study analyzing monotherapy with saridegib had negative results.47 The reduction in splenomegaly was modest, symptoms (as evaluated by Myeloproliferative Neoplasm Symptom Assessment Form scores) did not improve significantly, and only 4 of 10 evaluated patients had minimal to modest decreases in bone marrow fibrosis after 3 months. Exploratory studies that evaluated markers of Hh signaling in this study did not show a reduction of Gli1 messenger RNA and protein levels, indicating that Gli1 might be activated by noncanonical Smo-independent (oncogenic) pathways in MF (Figure 1). Accumulating data indicate that Gli proteins can also be activated in an Smo-independent fashion by TGF-β,48,49 PDGF signaling,50,51 and AKT/PI3K pathways,52-57 all of which have also been reported to play an important role in JAK2V617F-mutated MPNs. In light of these studies, it is obvious that the regulation of Gli activity is subject to canonical Hh/Ptch/Smo-dependent signaling and is also controlled by integration of multiple oncogenic non-Hh signals. In the field of cancer, this concept has led to the hypothesis that Gli proteins are an information nexus in the regulation of cell fate, stemness, and oncogenesis. We found that Gli1 expression is significantly increased in stromal cells from MPN patients and in murine Gli1+ stromal cells in response to JAK2V617F-mutant cells. As outlined above, our data in a murine model demonstrate that pharmacologic targeting of Gli proteins with a small-molecule Gli inhibitor ameliorates bone marrow fibrosis in JAK2V617F MPNs. Thus, we propose that Gli proteins in hematopoietic and stromal cells in MF can be activated independently of canonical Hh signaling, which could potentially explain the poor response of Smo inhibitors in patients with PMF. Targeting abnormal Gli activation in fibrosis-causing cells and in the mutant hematopoietic clone might be an optimal strategy for treating MF because Gli and Hh signaling were shown to be dispensable for normal hematopoiesis in 2 back-to-back seminal articles published in 2009,58-60 which could potentially spare normal hematopoiesis while eliminating both malignant hematopoietic cells and activated fibrosis-driving cells. Clearly, future studies are needed to dissect the complex role of canonical and noncanonical Hh signaling in MF and to understand the altered gene expression patterns in fibrosis-driving cells during the fibrotic transformation.
Targeting Hh-Gli signaling in MF. In canonical Hh-Gli signaling, 1 of the 3 ligands, Indian (Ihh), sonic (Shh), or desert Hh (Dhh), binds to the receptor patched 1 (Ptch1) which subsequently releases its tonic inhibition on the transmembrane protein smoothened (Smo). Smo then activates the Gli family zinc finger transcription factors, which translocate into the nucleus and activate expression of Hh target genes. Canonical Hh activation can be inhibited by various Smo inhibitors such as IPI-926 (saridegib), LDE-225 (sonidegib), and PF-0444991 among others. Recent evidence indicates that Gli proteins can also be activated noncanonically and directly, independent of Ptc1 and Smo. Various pathways such as EGF, AKT/PI3K, PDGF, and TGF-β signaling have been reported to directly activate Gli proteins. A direct inhibition of Gli proteins by small-molecule compounds such as Gli antagonist 61 (GANT61) have the advantage of blocking Gli proteins independent of their mechanism of activation.
Targeting Hh-Gli signaling in MF. In canonical Hh-Gli signaling, 1 of the 3 ligands, Indian (Ihh), sonic (Shh), or desert Hh (Dhh), binds to the receptor patched 1 (Ptch1) which subsequently releases its tonic inhibition on the transmembrane protein smoothened (Smo). Smo then activates the Gli family zinc finger transcription factors, which translocate into the nucleus and activate expression of Hh target genes. Canonical Hh activation can be inhibited by various Smo inhibitors such as IPI-926 (saridegib), LDE-225 (sonidegib), and PF-0444991 among others. Recent evidence indicates that Gli proteins can also be activated noncanonically and directly, independent of Ptc1 and Smo. Various pathways such as EGF, AKT/PI3K, PDGF, and TGF-β signaling have been reported to directly activate Gli proteins. A direct inhibition of Gli proteins by small-molecule compounds such as Gli antagonist 61 (GANT61) have the advantage of blocking Gli proteins independent of their mechanism of activation.
Functionally reprogrammed hematopoietic niche in MPNs and implications for graft function after allo-HSCT
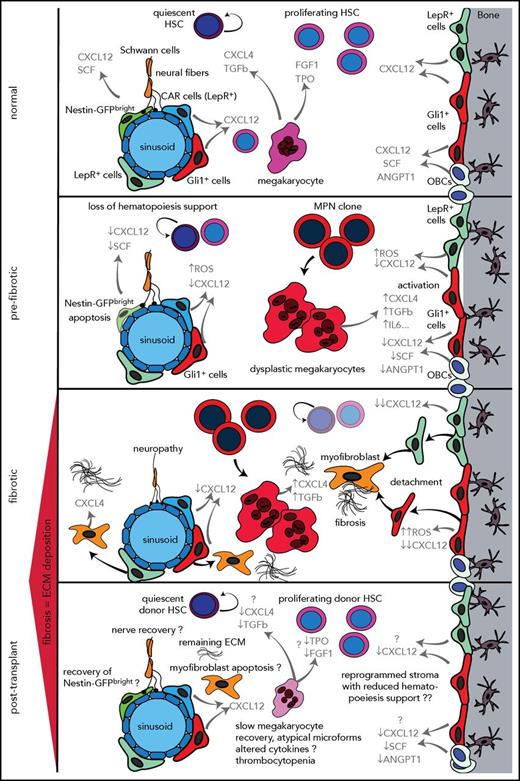
It was noted more than 30 years ago that MF is associated with delayed hematopoietic reconstitution after allo-HSCT. By using reduced-intensity conditioning (RIC) regimens, HSCT has become a well-established curative treatment for high-risk MF patients with acceptable posttransplant nonrelapse mortality, but 1 of the complications is still poor graft function. It has been hypothesized that the relatively high incidence of poor graft function observed in patients with MF after allo-HSCT may be caused by splenomegaly before and after transplantation, which may result in sequestration of transplanted CD34+ cells and sequestration of peripheral blood cells.61 There is also growing evidence that molecular genetics influence outcome for patients with MF. Kröger et al62 demonstrated in a study that screened 169 patients with MF that ASXL1 and IDH2 are independent risk factors for shorter progression-free survival. In a cohort of 1514 patients with myelodysplastic syndrome (MDS) before transplantation, Lindsley et al63 showed that JAK2 mutations were associated with a higher rate of death without relapse but not a higher risk of relapse, regardless of conditioning intensity. The mechanism of these effects of JAK2 mutations is unknown, but it might be associated with the influence of JAK2 mutations on altering the bone microenvironment (Figure 2). In general, significant bone marrow fibrosis is reported in 10% to 20% of patients with MDS and is not necessarily linked to mutations in CALR or JAK2. These data suggest that mutations can influence the bone marrow niche and graft function independent of fibrosis. However, once fibrosis is established and the stem cell niche is significantly remodeled (this is true for MDS and MPN), overall survival is significantly reduced even in patients who underwent allo-HSCT, suggesting that the effect on the microenvironment and stem cell niche is not completely reversible after allo-HSCT (Figure 2).64-66 In summary, these data on the influence of fibrosis and mutations on outcome after allo-HSCT indicate that the hematopoiesis-supporting HSC niche (bone marrow microenvironment) might be altered in the presence of mutated HSCs and that these changes even persist after HSCT in terms of a functionally reprogrammed stroma (Figure 2).
Stepwise reprogramming of the bone marrow niche with particular focus on stromal cell populations implicated as playing a role in MF. In the bone marrow (BM) niche under normal (healthy) conditions, HSC function is tightly controlled by a specialized microenvironment comprising sympathetic neurons, Nes+, LepR+, and Gli1+ MSCs and OBCs. These stromal cell subsets, megakaryocytes,77,83 growth factors, and cytokines control the proliferation and maintenance of the HSCs. In prefibrotic stages of PMF, hematopoietic stem and progenitor cells acquire genetic alterations in MPNs (MPN clone). The MPN clone gives rise to mutant dysplastic megakaryocytes that contribute to a proinflammatory environment that damages sensitive elements of the microenvironment such as Nes+ MSCs, Schwann cells, and their associated nerve terminals and thus contributes to the loss of normal hematopoiesis. Megakaryocytes derived from the malignant MPN clone significantly contribute to the reprogramming of stromal cells. Stromal cell subsets lose their hematopoiesis support (eg, loss of CXCL12 and increased cellular stress through accumulation of ROS and maintain a profibrotic environment that favors the malignant hematopoietic clone and leads to a vicious circle that results in bone marrow fibrosis and continuous production of extracellular matrix proteins, inflammation, loss of normal hematopoiesis, and expansion of the MPN clone. In the fibrotic phase, stromal cell subsets acquire an abnormal phenotype and start proliferating, detaching, and migrating from their normal niches; they can be found in abundance in the marrow cavity where they differentiate into myofibroblasts under the influence of CXCL4 secreted by megakaryocytes. They also start expressing CXCL4, which further negatively affects normal hematopoiesis and induces myofibroblast differentiation. The inflammatory environment created by the MPN clone, mutant megakaryocytes, and functionally and transcriptionally reprogrammed stromal cells (myofibroblasts) leads to a self-reinforcing niche. It remains unclear how this self-reinforcing niche is interrupted through HSCT. The posttransplant phase raises numerous open questions as indicated by the question marks. Detailed histopathologic studies on posttransplant bone marrow biopsies indicate resolution of bone marrow fibrosis but it remains an open question whether the reprogrammed stroma in bone marrow fibrosis regains its function and whether the stromal reprogramming is a reversible process after HSCT. Megakaryocytes in posttransplant bone marrows show atypical microforms exhibiting a dysplastic aspect that might be the result of an altered interaction between stromal cells (recipient bone marrow) and donor megakaryocytes that have not completely recovered their functionality. This disturbed interaction can in turn negatively affect regenerating hematopoiesis, in line with delayed hematopoiesis recovery in patients with bone marrow fibrosis after HSCT. Another open question is whether nerve fibers and nestin+ stromal cell subsets are able to recover after HSCT. Future studies will shed light on regenerative processes in the bone marrow microenvironment after HSCT and answer the question of whether the reprogrammed self-reinforcing niche in bone marrow fibrosis is reversible. Cytokines and growth factors are highlighted in gray. ANGPT1, angiopoietin; CAR, cxcl12-abundant reticular cells; CXCL12, C-X-C motif chemokine 12; CXCL4, C-X-C motif chemokine 4 (or platelet factor 4); ECM, extracellular matrix; FGF1, fibroblast growth factor 1; GFP, green fluorescent protein; IL6, interleukin 6 SCF, stem cell factor; TPO, thrompoietin.
Stepwise reprogramming of the bone marrow niche with particular focus on stromal cell populations implicated as playing a role in MF. In the bone marrow (BM) niche under normal (healthy) conditions, HSC function is tightly controlled by a specialized microenvironment comprising sympathetic neurons, Nes+, LepR+, and Gli1+ MSCs and OBCs. These stromal cell subsets, megakaryocytes,77,83 growth factors, and cytokines control the proliferation and maintenance of the HSCs. In prefibrotic stages of PMF, hematopoietic stem and progenitor cells acquire genetic alterations in MPNs (MPN clone). The MPN clone gives rise to mutant dysplastic megakaryocytes that contribute to a proinflammatory environment that damages sensitive elements of the microenvironment such as Nes+ MSCs, Schwann cells, and their associated nerve terminals and thus contributes to the loss of normal hematopoiesis. Megakaryocytes derived from the malignant MPN clone significantly contribute to the reprogramming of stromal cells. Stromal cell subsets lose their hematopoiesis support (eg, loss of CXCL12 and increased cellular stress through accumulation of ROS and maintain a profibrotic environment that favors the malignant hematopoietic clone and leads to a vicious circle that results in bone marrow fibrosis and continuous production of extracellular matrix proteins, inflammation, loss of normal hematopoiesis, and expansion of the MPN clone. In the fibrotic phase, stromal cell subsets acquire an abnormal phenotype and start proliferating, detaching, and migrating from their normal niches; they can be found in abundance in the marrow cavity where they differentiate into myofibroblasts under the influence of CXCL4 secreted by megakaryocytes. They also start expressing CXCL4, which further negatively affects normal hematopoiesis and induces myofibroblast differentiation. The inflammatory environment created by the MPN clone, mutant megakaryocytes, and functionally and transcriptionally reprogrammed stromal cells (myofibroblasts) leads to a self-reinforcing niche. It remains unclear how this self-reinforcing niche is interrupted through HSCT. The posttransplant phase raises numerous open questions as indicated by the question marks. Detailed histopathologic studies on posttransplant bone marrow biopsies indicate resolution of bone marrow fibrosis but it remains an open question whether the reprogrammed stroma in bone marrow fibrosis regains its function and whether the stromal reprogramming is a reversible process after HSCT. Megakaryocytes in posttransplant bone marrows show atypical microforms exhibiting a dysplastic aspect that might be the result of an altered interaction between stromal cells (recipient bone marrow) and donor megakaryocytes that have not completely recovered their functionality. This disturbed interaction can in turn negatively affect regenerating hematopoiesis, in line with delayed hematopoiesis recovery in patients with bone marrow fibrosis after HSCT. Another open question is whether nerve fibers and nestin+ stromal cell subsets are able to recover after HSCT. Future studies will shed light on regenerative processes in the bone marrow microenvironment after HSCT and answer the question of whether the reprogrammed self-reinforcing niche in bone marrow fibrosis is reversible. Cytokines and growth factors are highlighted in gray. ANGPT1, angiopoietin; CAR, cxcl12-abundant reticular cells; CXCL12, C-X-C motif chemokine 12; CXCL4, C-X-C motif chemokine 4 (or platelet factor 4); ECM, extracellular matrix; FGF1, fibroblast growth factor 1; GFP, green fluorescent protein; IL6, interleukin 6 SCF, stem cell factor; TPO, thrompoietin.
Recent studies using different models of hematopoietic malignancies associated with bone marrow fibrosis were able to mechanistically elucidate how malignant mutated hematopoietic cells and proinflammatory cytokines activate distinct subsets of stromal cells in the bone marrow, support their fibrotic and secretory activity, and influence their reduced hematopoiesis-supporting capacity.9,67,68
Two recent studies show that malignant hematopoietic cells in murine models of MPN67 and acute myeloid leukemia69 can damage nestin+ (Nes+) MSCs and adjacent nerve cells. In these models, the hematologic malignancy created neuropathic changes in the bone marrow niche, which affected the activity of MSCs and altered the function of the HSC niche. In the presence of malignant hematopoietic cells, an abnormal population of Nes+ MSCs expanded with skewed osteoblastic differentiation and downregulated expression of many HSC retention factors, including CXCL12 and stem cell factor, indicating loss of hematopoiesis support of the HSC niche in MPN and AML. Thus, these studies indicate that the MPN- and acute myeloid leukemia–induced neuropathy promotes the development of a self-reinforcing malignant niche that favors disease development and progression at the expense of normal HSC maintenance. Schepers et al68 demonstrated that osteoblastic lineage cells (OBCs) derived from multipotent stromal cells expand in the presence of malignant hematopoietic cells, which results in matrix production and trabecular thickening. They demonstrated that BCR-ABL+ malignant hematopoietic cells stimulate MSCs to proliferate and to adopt an abnormal differentiation program that results in the overproduction of functionally altered OBCs, which accumulate in the bone marrow cavity as inflammatory myelofibrotic cells. The authors investigated the molecular basis of this observation and found that leukemia-conditioned OBCs aberrantly expressed several key molecules known to coordinate niche function and HSC retention, including the chemokine CXCL12, n-cadherin, stem cell factor, angiopoietin-1 (ANGPT1), and TGF-β1 and -β2. The data indicate that malignant hematopoietic cells diminish the hematopoietic supportive capacity of bone marrow stromal and niche cells indirectly through changes in the physical and cytokine environment. In line with these studies, our own study on Gli1+ cells and the analysis of LepR+ cells demonstrated altered expression of many HSC-regulatory genes and cytokines, including a broad downregulation of HSC retention factors, especially CXCL12 in MF, indicating transcriptional reprogramming from hematopoiesis support to inflammatory profibrotic programs.
One of the common denominators in solid organ fibrosis and MF seems to be inflammation, which is induced either by tissue injury (or in the bone marrow, eg, by radiation, certain drugs, or infections) or by the presence of a malignant hematopoietic clone. In contrast to acute inflammatory reactions that are characterized by rapidly resolving vascular changes, edema, and neutrophilic infiltration, pathogenic fibrosis is typically a result of chronic inflammatory reactions defined as responses that persist for several weeks or months and in which inflammation and repair processes occur simultaneously. Despite having obvious etiologic and clinical distinctions, most chronic fibrotic disorders have in common a persistent irritant that sustains the production of growth factors, proteolytic enzymes, angiogenic factors, and fibrogenic cytokines, which together stimulate the deposition of connective tissue elements that progressively remodel and destroy normal tissue architecture.9,68,70 In particular, oxidative stress and the antioxidant system seem to be crucial modulators of processes such as TGF-β signaling and metabolic homeostasis, which play critical roles in the development and persistence of fibrosis. Reactive oxygen species (ROS) levels contribute to the fibrotic process either directly or indirectly via enhanced inflammation. Conversely, fibrosis and inflammation might further increase ROS formation or stimulate the production of cytokines and growth factors in a vicious circle.71,72 In MF, ROS expression is significantly increased in fibrosis-driving stromal cells (Figure 2).9 Our data indicate that the inhibition of Gli/Hh signaling might also influence the inflammatory reaction in Gli1+ cells. Endothelin 1, a potent vasoconstrictor, plays a role in solid organ fibrosis by stimulating ROS production. Treatment with the Gli inhibitor GANT61 in MF significantly reduced the expression of endothelin 1, CXCL4, and MMP9 in Gli1+ fibrosis-driving cells, all important mediators of tissue repair, inflammation, and fibrosis. It also reduced the increased PI3K and STAT5 expression in JAK2V617F-mutant hematopoietic cells, indicating that Hh-Gli inhibition can modulate the inflammatory reaction in bone marrow fibrosis and interrupt the vicious circle of inflammation and myofibroblast differentiation. Future studies will dissect the role of Hh-Gli inhibition on the inflammatory profibrotic program in MF.
The systematic dissection of events that convert the bone marrow from a nurturing environment to a hostile, fibrotic niche may hold important translational value for the treatment of hematopoietic neoplasms. The dynamic between malignant hematopoietic cells and stromal cells describes an active process in which reprogramming of niche components by neoplastic cells favors MF and leukemic proliferation while compromising normal hematopoiesis. Microenvironmental contributions to leukemic drug resistance have long been appreciated, and our studies suggest that, similarly, MPN-associated bone marrow dysfunction is an elegantly orchestrated component of the disease progression and fibrotic transformation rather than a simple case of physical competition for available space and resources within the bone marrow. The mechanisms by which the neoplastic cells sustain this microenvironment, which favors malignant hematopoiesis at the expense of normal hematopoiesis, remain enigmatic, but studies are accumulating that show a central role for both inflammation and megakaryocytes and platelets in this process.
Mechanisms in the transcriptional and functional reprogramming of stromal cells in MPNs
A considerable amount of experimental evidence in solid organ fibrosis implies that platelets play a central role in the fibrotic process, mainly by releasing profibrotic mediators. Platelets tend to accumulate and activate at sites of tissue injury, the initiating event in most cases of solid fibrosis. Platelets are a major cellular source for TGF-β and PDGFs, 2 of the most powerful profibrotic mediators in the human body. In the bone marrow, a central role has been attributed to megakaryocytes. In all MPNs, megakaryocytes proliferate, have pathognomonic multilobulated nuclei, and exhibit clustering in the marrow. These megakaryocytes have abnormal location of p-selectin on their intracytoplasmic vacuoles and a demarcation membrane system that leads to the increased emperipolesis (the passage of a cell into the cytoplasm of another cell) of neutrophils. The neutrophils release their enzymes in the megakaryocytes, which leads to the release of cytokines such as TGF-β, PDGFs, and fibroblast growth factor from their α granules. In the past, the standing hypothesis was that these growth factors then stimulate the stromal cell population to cause fibrosis and the endothelial cells to cause neoangiogenesis. The significant breakthrough of identifying Gli1+ and LepR+ cells as fibrosis-driving cells in the bone marrow confirmed the central role of megakaryocytes in transcriptionally reprogramming hematopoiesis-supporting cells to fibrosis-driving cells. As mentioned above, in LepR+ cells PDGFRα is critical in the development of bone marrow fibrosis because a LepR+ cell–specific knockout of PDGFRα resulted in greatly reduced reticulin fibrosis. Conversely, activation of the PDGFRα pathway in bone marrow LepR+ cells led to expansion of these cells and extramedullary hematopoiesis as features of PMF. Differential gene expression analysis in Gli1+ cells in bone marrow fibrosis demonstrated that megakaryocyte-associated genes were significantly upregulated, in particular the chemokine C-X-C motif ligand 4 (CXCL4) and the arachidonate lipoxygenase enzyme ALOX12, which have been implicated as playing a role in fibrosis73-76 and HSC regulation77 and are linked to megakaryocyte, platelet, and stromal cell biology.78,79
Future studies will focus on dissecting the interaction between megakaryocytes derived from the MPN clone and fibrosis-driving cells. An important question to be answered is whether the interaction between dysplastic megakaryocytes and fibrosis-driving stromal cells functions mainly via cytokines and growth factors or whether there is heterocellular transfer of material (eg, through mitochondrial transfer or microvesicles).80,81 The interaction between dysplastic megakaryocytes and stromal cells is a promising therapeutic target, and previous studies demonstrated that targeting activated pathways in fibrosis-driving cells or the impaired differentiation of megakaryocytes in MPN through Aurora kinase inhibition, for example, can ameliorate bone marrow fibrosis. This concept was proven in a murine model by treatment with MLN8237, a selective Aurora kinase A inhibitor.82 MLN8237 promoted polyploidization and differentiation of megakaryocytes with PMF-associated mutations and had potent antifibrotic and antitumor activity in vivo in mouse models of PMF.
Conclusions and future directions
The recent discovery of MSC populations as the cellular origin of fibrosis-driving myofibroblasts is an important step toward better understanding of the bone marrow niche in MF and development of novel targeted therapeutics. Emerging techniques that allow single-cell characterization will help researchers understand the heterogeneity of MSCs in homeostasis and disease. It remains an open question whether the reported Gli1+ and LepR+ cells overlap or whether multiple distinct myofibroblast precursors exist in the bone marrow. Although recent studies shed light on some mechanisms of MSC activation and expansion, more studies are needed to elucidate the exact mechanisms of how the malignant hematopoietic cells recruit and reprogram MSCs toward fibrosis-driving myofibroblasts. Targeting Gli proteins directly or pharmacologic intervention in the PDGFRα signaling pathway might be interesting novel therapeutic options. However, additional studies are needed to identify novel and specific Gli inhibitors and to test these as stand-alone therapy or in a combinatory regime with existing JAK inhibitors.
Acknowledgments
This work was supported by a John Goldman Clinical Research grant from the European Hematology Association, a research grant from the MPN Research Foundation (MPNRF; 2017 MPNRF/LLS Award), a KWF Kankerbestrijding young investigator grant (11031/2017-1 [Bas Mulder Award] from the Dutch Cancer Foundation), a Celgene research grant (DEU-077), and a grant from the European Research Council (ERC) (deFIBER; ERC-StG 757339) (all to R.K.S.). Additional support was provided by grants from the German Research Foundation (KR-4073/3-1, SCHN1188/5-1, SFB/TRR57, SFB/TRR219), an ERC grant (ERC-StG 677448), a START grant from the Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen University (101/15), a grant from the State of Northrhinewestfalia (Return to NRW), a grant from the Interdisciplinary Centre for Clinical Research within the Faculty of Medicine at the RWTH Aachen University (IZKF O3-11), and a research grant from MPNRF (2017 MPNRF/LLS Award) (all to R.K.).
Authorship
Contribution: R.K. and R.K.S prepared the figures and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rebekka K. Schneider, Erasmus MC Cancer Institute, Faculty Building 1330e, Wytemaweg 80, 3015CN Rotterdam, The Netherlands; e-mail: r.k.schneider@erasmusmc.nl; and Rafael Kramann, University Hospital, RWTH Aachen, Department of Nephrology and Clinical Immunology, Pauwelstr 30, 52074 Aachen, Germany; e-mail: rkramann@gmx.net.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal