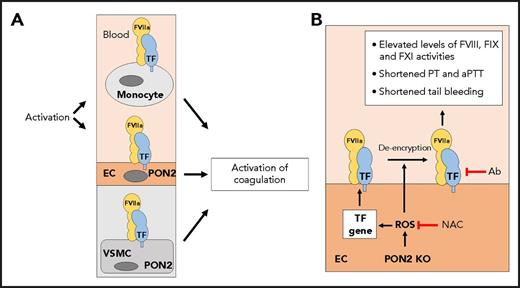
In this issue of Blood, Ebert et al conclude that endothelial cell (EC) tissue factor (TF) activity induces a prothrombotic state in mice that lack the antioxidant paraoxonase-2 (PON2).1
(A) TF is constitutively expressed by vascular smooth muscle cells (VSMCs) and activated monocytes and ECs. PON2 is expressed by macrophages, VSMCs, and ECs. (B) Loss of PON2 leads to increased levels of reactive oxygen species (ROS) in ECs and de-encryption of TF. In addition, increased levels of ROS may induce TF gene expression. Increased levels of EC TF activity are proposed to mediate the prothrombotic state in PON2−/− mice, which was assessed by measuring plasma FVIII, FIX, and FXI activities, prothrombin time (PT), activated partial thromboplastin time (aPTT), and tail bleeding. The antioxidant N-acetylcysteine (NAC) and an anti-mouse antibody (Ab) normalize the prothrombotic state in PON2−/− mice. KO, knockout.
(A) TF is constitutively expressed by vascular smooth muscle cells (VSMCs) and activated monocytes and ECs. PON2 is expressed by macrophages, VSMCs, and ECs. (B) Loss of PON2 leads to increased levels of reactive oxygen species (ROS) in ECs and de-encryption of TF. In addition, increased levels of ROS may induce TF gene expression. Increased levels of EC TF activity are proposed to mediate the prothrombotic state in PON2−/− mice, which was assessed by measuring plasma FVIII, FIX, and FXI activities, prothrombin time (PT), activated partial thromboplastin time (aPTT), and tail bleeding. The antioxidant N-acetylcysteine (NAC) and an anti-mouse antibody (Ab) normalize the prothrombotic state in PON2−/− mice. KO, knockout.
Because of its potent procoagulant activity, TF gene expression and activity are tightly regulated to maintain hemostasis while preventing thrombosis. TF is constitutively expressed by perivascular cells, such as vascular smooth muscle cells (see figure panel A).2 In contrast, unstimulated blood monocytes and ECs do not express TF. However, they express TF after activation as part of the host defense system. TF activity is also regulated posttranslationally with several proposed mechanisms of de-encryption, including interaction with phosphatidylserine and oxidation of an allosteric disulfide bond (see figure panel B).2
The PON family of antioxidants consists of 3 members: PON1, PON2, and PON3.3 PON1 and PON3 are expressed by the liver and bind to high density lipoprotein and prevent its oxidation. In contrast, PON2 is expressed by macrophages, vascular smooth muscle cells, and ECs (see figure panel A).3 Interestingly, PON2-deficient mice developed atherosclerosis when fed a Western diet, and this was attributed to a deficiency of PON2 in macrophages.4
Ebert and colleagues observed numerous abnormalities in PON2−/− mice, including increased reactive oxygen species in the aorta; increased levels of interleukin-6 (IL-6) in the plasma; elevated plasma levels of factor VIII (FVIII), FIX, and FXI activities; shortened prothrombin time; shortened activated partial thromboplastin time; and a shortened tail bleeding time. Furthermore, they found that platelets expressed PON2, and deficient cells had increased reactive oxygen species, increased volume, and increased binding of annexin V. In vitro studies with primary lung ECs showed increased basal levels of a variety of inflammatory mediators and a decrease in the anticoagulant TF pathway inhibitor. Surprisingly, the expression of other markers of EC activation, such as ICAM-1, E-selectin, or VCAM-1, were not increased. Primary lung PON2−/− ECs expressed TF messenger RNA and protein at similar levels to those in wild-type cells but had increased levels of TF activity (see figure panel B).
Because PON2 is expressed by macrophages, vascular smooth muscle cells, and ECs, it was important to determine which cell types contributed to the different phenotypes in PON2−/− mice. Importantly, an anti-mouse TF antibody normalized the prothrombotic phenotype, which indicates that the prothrombotic phenotype was due to TF. Bone marrow transplantation experiments showed that the prothrombotic phenotype tracked with PON2 deficiency in nonhematopoietic cells. However, there was significant variability in the hemostatic parameters in these experiments. It is also surprising that platelets did not contribute to the shortened tail bleeding. Finally, re-expression of PON2 in EC and hematopoietic cells normalized the prothrombotic phenotype. These results led the authors to conclude that the prothrombotic phenotype in PON2−/− mice is the result of an increase in EC TF activity but not the result of changes in TF expression in hematopoietic cells or vascular smooth muscle cells.
Unstimulated ECs express several anticoagulants, such as TF pathway inhibitor, EC protein C receptor, and thrombomodulin, to prevent activation of coagulation. Activated ECs become procoagulant because of the downregulation of EC protein C receptor and thrombomodulin and the induction of TF. Although TF expression is readily detectable in cultured human and murine ECs, it has been difficult to demonstrate in vivo.2 The limited examples of TF staining of ECs include splenic microvascular ECs and arterial branching areas in septic baboons, ECs of the glomerulus of endotoxemic mice, and ECs in the lungs of sickle cell mice.5-8 It is possible that some or all of this TF staining is a result of the binding of TF-positive monocyte-derived microvesicles to activated ECs.
We and others have used genetic approaches to study the role of EC TF in the activation of coagulation in different diseases. We found that deletion of EC TF did not affect the activation of coagulation in a mouse endotoxemia model.9 Similarly, deletion of EC TF did not affect the activation of coagulation but reduced IL-6 expression in a mouse model of sickle cell disease.10 Song and colleagues7 generated mice with inducible expression of a degradation resistant form of IκBα in ECs and found that it blocked the induction of TF in ECs in the glomerulus of endotoxemic mice. They could not determine the impact of preventing EC TF expression on the activation of coagulation because the mutant form of IκBα also prevented the downregulation of EC protein C receptor and thrombomodulin.
The study by Ebert et al has several limitations. One must be cautious when extrapolating data from in vitro studies to explain a complex phenotype in mice. For instance, primary lung ECs from both PON2−/− and wild-type mice expressed high TF levels, which suggests that the cells were activated. Indeed, no direct data were presented to show EC TF expression in PON2−/− mice. The role of EC TF in the prothrombotic state in PON2−/− mice could be directly examined by crossing PON2−/− mice with mice that lacked EC TF. The model presented by Ebert et al shows that the absence of PON2 only leads to de-encryption of EC TF. However, it seems that increased reactive oxygen species may also induce EC TF gene expression (see figure). The prothrombotic state of PON2−/− mice was assessed by using plasma-based coagulation assays, which are not routinely used to monitor activation of coagulation. Typically, a prothrombotic state in mice is associated with increased levels of thrombin-antithrombin complexes and decreased levels of fibrinogen and fibrin deposition. Interestingly, levels of thrombin-antithrombin complexes were not increased in PON2−/− mice. Furthermore, the prothrombotic state was functionally evaluated by using tail bleeding rather than thrombosis models. The current data do not exclude the possibility that PON2 deficiency in ECs leads to increased vascular permeability that would expose subendothelial TF to blood and contribute to the prothrombotic state. Finally, the investigators acknowledge the fact that a reduction of EC protein C receptor and thrombomodulin could also contribute to the prothrombotic state observed in PON2−/− mice. Although the study by Ebert et al is provocative, it seems premature to suggest that PON2 and EC TF activity represent new targets for anticoagulant and anti-inflammatory strategies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.